Durham E-Theses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Local Government Boundary Commission for England Report No
Local Government Boundary Commission For England Report No. 71 LOCAL GOVERNMENT BOUNDARY COMMISSION FOR ENGLAND REPORT NO. LOCAL GOVERNMENT BOUNDARY COMMISSION FOR ENGLAND CHAIRMAN Sir Edmund Compton, GCB.KBE. DEPUTY CHAIRMAN Mr J M Rankin.QC. MEMBERS The Countess Of Albemarle, DBE. Mr T C Benfield. Professor Michael Chisholjn. Sir Andrew Wheatley,CBE. Mr F B Young, CBE. To the Rt Hon Roy Jenkins, MP Secretary of State for the Home Department PROPOSALS FOR REVISED EI£CTORAL ARRANGEMENTS FUR THE BOROUGH OF GEDLING IN THE COUNT*/ OF NOTTINGHAMSHIRE 1. We, the Local Government Boundary Commission for England, having carried out our initial review of the electoral arrangements for the borough of Gedling in accordance with the requirements of section 63 of and Schedule 9 to the Local Government Act 1972, present our proposals for the future electoral arrangements for that borough* 2. In accordance with the procedure laid down in section 60 (l) and (2) of the 1972 Act, notice was given on 18 January 1974 that we were to undertake this review. This was incorporated in a consultation letter addressed to the Gedling Borough Council, copies of which were circulated to the Nottinghamshire County Council, Parish Councils in the district, the Members of Parliament for the constituencies concerned and the headquarters of the main political parties* Copies were also sent to the editors of local newspapers circulating in the area and of the Local Government press and to the local radio broadcasting station* Notices inserted in the local press announced the start of the review and invited comments from members of the public and from any interested bodies. -

The London Gazette, 27 March, 1923
2344. THE LONDON GAZETTE, 27 MARCH, 1923. (Derbyshire Lines), bridge carrying the Scottish Railway (Blackwell Branch) over road from Tibshelf to Sawpit Lane over Fordbridge Lane. -the London and North Eastern Railway Parish of Tibshelf— (Tibshelf Colliery Branch). Bridges carrying the London, Midland (D) Roads under the following bridges:— and Scottish Railway over the roads from Tibshelf to Westhouses and from Tibshelf In the urban district of Sutton-in-Ash- to Morton, bridge carrying the London, fieldt— Midland and Scottish Railway (Tibshelf Bridge carrying the London and North and Pleasley) over Newton Lane, bridges Eastern Railway (Mansfield Railway) over carrying the London and North Eastern Coxmoor Road. Railway (Derbyshire Lines) over Newton Lane and Pit Lane, bridge carrying the In the urban district of Kirkby-in-Ash- London and North Eastern Railway (Tib- field:— shelf Colliery Branch) over Sawpit Lane. Bridge carrying the London, Midland and (E) Railways: — Scottish Railway (Mansfield and Pinxton) over Mill Lane, bridge carrying the In the urban district of Sutton-in-Ash- •London, Midland and Scottish Railway field: — (Bentinck Branch) over Park Lane, bridge Level crossings of the London, Midland carrying the London and North Eastern and Scottish Railway (Nottingham and Railway (Langton Colliery Branch) over Mansfield) in Station Road and Coxmoor the road from Kirkby-in-Ashfield to Road. - Finxton, bridges carrying^ mineral rail- ways at Kirkby Colliery over Southwell In the urban district of Huthwaiter — Lane, bridge carrying mineral railway at Level crossing of mineral railway from Bentinck Colliery over Mill Lane. New Hucknall Colliery in Common Road. In the urban district of Kirkby-in-Ash- In the rural district of Basford: — field: — Parish of Linby— Bridge carrying the London and NortL .Level crossings of the London, Midland Eastern Railway over the road from and Scottish Railway (Nottingham and Linby to Annesley. -
10/02/2021 MEMBERS INTERESTS Page 1
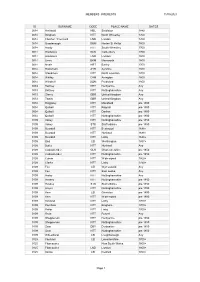
MEMBERS INTERESTS 11/09/2021 ID SURNAME CODE PLACE NAME DATES 0014 Archbold NBL Embleton 1840 0014 Bingham NTT North Wheatley 1700 0014 Fletcher / Fruchard LND London 1700 0014 Goodenough SOM Norton St Phillip 1800 0014 Hardy NTT South Wheatley 1700 0014 Holdstock KEN Canterbury 1700 0014 Holdstock LND London 1800 0014 Lines BKM Marsworth 1800 0014 Neale HRT Barley 1700 0014 Robertson AYR Ayrshire 1800 0014 Steedman NTT North Leverton 1700 0014 Whitby CAM Arrington 1800 0014 Windmill SOM Prudsford 1800 0033 Bettney DBY Derbyshire Any 0033 Bettney NTT Nottinghamshire Any 0033 Storey GBR United Kingdom Any 0033 Twells GBR United Kingdom Any 0034 Baggaley NTT Mansfield pre 1800 0034 Quibell NTT Ragnall pre 1800 0034 Quibell NTT Darlton pre 1800 0034 Quibell NTT Nottinghamshire pre 1800 0109 Askey NTT Nottinghamshire pre 1850 0109 Askey STS Staffordshire pre 1850 0109 Beardall NTT Bestwood 1688+ 0109 Beardall NTT Hucknall 1688+ 0109 Beardall NTT Linby 1688+ 0109 Bird LEI Worthington 1857+ 0109 Butler NTT Hucknall Any 0109 Cadwallender GLS Gloucestershire pre 1850 0109 Cadwallender NTT Nottinghamshire pre 1850 0109 Camm NTT Widmerpool 1800+ 0109 Clarke NTT Linby 1750+ 0109 Fox LEI Wymeswold Any 0109 Fox NTT East Leake Any 0109 Harby NTT Nottinghamshire Any 0109 Haskey NTT Nottinghamshire pre 1850 0109 Haskey STS Staffordshire pre 1850 0109 Hayes NTT Nottinghamshire pre 1700 0109 Kem LEI Grimston pre 1800 0109 Kem NTT Widmerpool pre 1800 0109 Kirkland NTT Linby 1700+ 0109 Parnham NTT Bingham 1700+ 0109 Potter NTT Linby 1700+ 0109 Rose NTT Bulwell -

Land Bounded by Main Street, Jennison Street and Linby Street , Nottingham
Public Document Pack ADDITIONAL / TO FOLLOW AGENDA ITEMS This is a supplement to the original agenda and includes reports that are additional to the original agenda or which were marked ‘to follow’. NOTTINGHAM CITY COUNCIL PLANNING COMMITTEE Date: Wednesday, 22 March 2017 Time: 2.30 pm Place: Ground Floor Committee Room - Loxley House, Station Street, Nottingham, NG2 3NG Governance Officer: Catherine Ziane-Pryor Direct Dial: 0115 8764298 AGENDA Pages d LAND BOUNDED BY MAIN STREET, JENNISON STREET AND 3 - 22 LINBY STREET , NOTTINGHAM This page is intentionally left blank Agenda Item 4d WARDS AFFECTED: Bulwell Item No: PLANNING COMMITTEE 22nd March 2017 REPORT OF CHIEF PLANNER Land Bounded By Main Street, Jennison Street And Linby Street , Nottingham 1 SUMMARY Application No: 16/01552/PFUL3 for planning permission Application by: Plan A (North West) Limited on behalf of Lidl UK GmbH Proposal: Erection of Class A1 retail store, car park and servicing areas, access and associated works following demolition of existing buildings and structures The application is brought to Committee because it is a major application on a prominent site where there are important land-use considerations. To meet the Council's Performance Targets this application should have been determined by 3rd October 2016. An extension of time has been agreed until 30th March 2017. 2 RECOMMENDATIONS 2.1 Subject to there being no additional material matters arising from the response of the Environment Agency, the power to grant planning permission subject to the indicative -

Appendix Nottinghamshire Green Estate Development Strategy
Appendix 1 Green Estate Sites (04/2014) Site Name Score Asset Area Public District Location Options (Hectares) Access KEY SITES Cotgrave Country Park 37 R 162.1 Part Rushcliffe Hollygate Lane, Cotgrave Daneshill Lakes 36 R 67.2 Yes Bassetlaw Daneshill Road, between Torworth & Lound Teversal & Silverhill Trails (8km w. Brierley Forest link) 33 R 11.4 Yes Ashfield Trail between county boundaries Pleasley and Woodend Moor Pond Wood 32 R 9.2 Yes Gedling Linby Ln, Papplewick (B6011) Southwell Trail (11.5 km incl Bilsthorpe arm) 31 R 27.6 Yes N&S Trail between Southwell & A614 & Bilsthorpe Cockglode and Rotary Woods LNR 29 R 14.9 Yes N&S Between Ollerton & Thoresby Colliery, Sherwood Heath Tippings Wood 29 R 51.2 Part N&S Warsop Lane, Blidworth/Rainworth (B6020) Great Northern Railway Path (1.7 km) 28 R 7.0 Yes Broxtowe Awsworth, Kimberley Ollerton Colliery (East) 28 R 58.2 Yes N&S Newark Rd, Ollerton Shirebrook Colliery North 28 R 79.1 Yes Mansfield Longster Lane, Sookholme (B6407) Shirebrook Colliery South 28 R 56.0 Yes Mansfield Wood Lane (off Bath Lane) Sookholme Rufford No. 1 (Rainworth Water) 26 R 60.5 No N&S Rufford Colliery Access Road, off Rainworth Bypass (A617) Dob Park 25 R 20.4 Yes Ashfield Washdyke Lane (west of Hucknall Bypass, A611) Harby-High Marnham SUSTRANS route (10km) 25 R 5.0 Yes N&S/Bassetlaw nr High Marnham power station to Lincs border Linby Trail (2km) 25 R 4.6 Yes Gedling Trail between Wighay Road, Linby to Newstead Shireoaks & Coachgap Green 25 R 29.6 Yes Bassetlaw Shireoaks Rd, Shireoaks Kimberley Green 24 R 7.2 -

Nottinghamshire Aviation Memorials
Nottinghamshire Aviation Memorials Aviation | Aviation Memorials in Nottinghamshire We love to commemorate our aviation heritage. In Nottinghamshire We Love To Commemorate Our Aviation Heritage The diversity of aviation memorial locations across the county is impressive. These memorials are not just at airfield sites, but they can also be found in churches, village halls, on city streets and at remote countryside locations. Some memorials are relatively new, whilst others can trace their origins back Nottinghamshire decades. These memorials, some of them raised through public subscription, reflect the lives of national figures like Albert Ball VC; whilst others are simpler marks of respect that have been erected thanks to the efforts of small groups of individuals. There are even sculptures and pub signs that highlight the county’s contribution to the development of significant aviation technologies. Collectively they play a part in helping to commemorate the county’s aviation heritage. Many individuals had travelled from around the world to air bases in Aviation Memorials Aviation | Nottinghamshire to train as World War II bomber crews. A common bond that joins most of these memorials together is that they commemorate the lives of brave individuals who were lost whilst learning these new skills; often in difficult weather conditions, a long way from home and in a relatively congested airspace, caused by having a lot of airfields so close together. For each of the memorials listed we have provided some background information about the crews involved and the circumstances of the crash; this is merely a snapshot of incidents that are recorded in more detail in books and on websites and we would encourage you to investigate them further. -

The Nottinghamshire County Council (Linby Avenue, Linby Grove and Linby Road, Hucknall) (Prohibition of Waiting) Traffic Regulation Order 2020 (4234)
The Nottinghamshire County Council (Linby Avenue, Linby Grove and Linby Road, Hucknall) (Prohibition of Waiting) Traffic Regulation Order 2020 (4234) The Nottinghamshire County Council ("the Council") in exercise of its powers under Sections 1(1) and (2), 2(1) to (3), 4(2), 9, 32, 35 and Part IV of Schedule 9 of the Road Traffic Regulation Act 1984 ("the 1984 Act"), Traffic Management Act 2004 (“the 2004 Act”), and by virtue of The Civil Enforcement of Parking Contraventions (County of Nottinghamshire) Designation Order 2008 (SI 2008 No. 1086) and of all other enabling powers and after consultation with the Chief Officer of Police for the Nottinghamshire Police Authority in accordance with Part III of Schedule 9 to the Act, hereby makes the following Order:- COMMENCEMENT This Order shall come into force for all purposes on the 7th day of February 2020 and may be cited as "The Nottinghamshire County Council (Linby Avenue, Linby Grove and Linby Road, Hucknall) (Prohibition of Waiting) Traffic Regulation Order 2020 (4234)” ARRANGEMENT OF SECTIONS Parts and Sections Allocated:- PART I (Waiting Control) Section 1: Prohibition and Restriction of Waiting (Articles 1, 2 and 3) PART VI Definitions of Category Letters GENERAL G1. All lengths of road specified in this Order are lengths of road at Hucknall in the District of Ashfield in the County of Nottinghamshire. G2. The prohibitions and restrictions imposed by this Order shall be in addition to and not in derogation of any restriction or requirement imposed by any regulations made or having effect as if made under the Act as amended aforesaid or by or under any other enactment. -

Nottinghamshire. 83
DIRECTORY.) NOTTINGHAMSHIRE. HUCKNA.LL TORKA.RD. 83 Elkington William, grocer & butcher, Cavendish street Hucknall Torkard Coffee Tavern & Institute· (James Cor- Emans Alfred George, picture frame maker, Portland road I den, manager), High street · Evans William, chimney sweep, Y orke street · Hucknall Torkard Dispatch & Leen V alley Mercury (pub. Evans William, hawker, Annesley road thurs.) (Henry Morley, printer & publisher), South street Evans William Jas. brush maker & ironmonger, Annesley road Hucknall Torkard Free Library (Albert Brecknock, librarian), Faulconbridge John, farmer, Bulwell Wood hall Market place Faulkner Frances (Mrs.), shopkeeper, Bestwood road Hucknall Torkard l\Iining Tool Manufacturing Co. tool Fellows John, shopkeeper, Annesley road makers, Annesley road & High street Foster Edward, insurance agent, Station terrace Hucknall Torkard Public Hall Co. Lim. William Burton, Foster Thomas, boot maker, Linby lane sec.), Watnall road Freeman Mary Ann (Mrs.), shopkeeper, Broom hill Humberstone Albert, shopkeeper, Byron street Gandy Hy. assist. clerk to Urban Dist. Council, Station rd Hunters' (The Teamt>n) Limited, gro!!ers, High street Gilliver William, furniture remover & agent for the Great Rutchinson Leonard Thos. stone & marble mason, West st Central Railway Co. Portland road Hutchinson Thomas, fruiterer, Annesley road · Godfrey Dan Enos, mineral water maker, Derbyshire lane Hyde Frank, joiner, Derbyshire lane Goodyear Thomas, shopkeepN, Cavendish street Ingle John, earthenware dealer, Victoria street Gosford Mildred (Mrs.), confectioner, Annesley road Jackson Henry, photographer, Washdyke lane Gough Benjamin, shopkeeper, George street J ackson J ames, shopkeeper, Hazel grove Granger William, farmer, The Common Jackson Ruth (Mrs.), grocer, Market place Green John, shopkeeper, West street Jackson Waiter, pork butcher, High street Green John, jun. shopkeeper, Annesley road James John, under manager, Hucknall Colliery Co. -

STATEMENT of PERSONS NOMINATED, NOTICE of POLL and SITUATION of POLLING STATIONS Election of a County Councillor
STATEMENT OF PERSONS NOMINATED, NOTICE OF POLL AND SITUATION OF POLLING STATIONS Nottinghamshire County Council Election of a County Councillor for Ashfields Division Notice is hereby given that: 1. A poll for the election of a County Councillor for Ashfields Division will be held on Thursday 6 May 2021, between the hours of 7:00 am and 10:00 pm. 2. One County Councillor is to be elected. 3. The names, home addresses and descriptions of the Candidates remaining validly nominated for election and the names of all persons signing the Candidates nomination paper are as follows: Names of Signatories Name of Description (if Home Address Proposers(+), Seconders(++) & Candidate any) Assentors FELTON (Address in The Conservative Flowers Carina(+) Flowers Cameron Ashfield District) Party Candidate Shaun A(++) LAMB (Address in Labour Party Fleet Natalie S(+) Fleet Elodie-Mai(++) Stefan Ashfield District) ZADROZNY 74 Sutton Road, Ashfield Gent Sarah(+) Barsby Kier(++) Jason Bernard Kirkby In Ashfield, Independents Nottinghamshire, Working All Year NG17 8GZ Round 4. The situation of Polling Stations and the description of persons entitled to vote thereat are as follows: Situation of Polling Station Station Number Ranges of electoral register numbers of persons entitled to vote thereat Mobile Unit at The Changing Rooms, Off Bluebell Wood Way, Sutton in Ashfield 1 ASH1-1 to ASH1-2087 The Changing Rooms, Off Bluebell Wood Way, Sutton In Ashfield 2 ASH2-1 to ASH2-874 Bentinck Miners Welfare, Sutton Road, Kirkby In Ashfield, Nottingham 3 LAR1-1 to LAR1-1876 Bentinck Miners Welfare, Sutton Road, Kirkby In Ashfield, Nottingham 4 LAR2-1 to LAR2-1126 Willetts Court Community Centre, Limb Crescent, Sutton In Ashfield 5 LEM1-1 to LEM1-1345/1 Willetts Court Community Centre, Limb Crescent, Sutton In Ashfield 6 LEM2-1 to LEM2-1510/1 5. -

Communities and Place Committee Thursday, 09 May 2019 at 10:30 County Hall, West Bridgford, Nottingham, NG2 7QP
Communities and Place Committee Thursday, 09 May 2019 at 10:30 County Hall, West Bridgford, Nottingham, NG2 7QP AGENDA 1 Minutes of last meeting held on 4 April 2019 3 - 6 2 Apologies for Absence 3 Declarations of Interests by Members and Officers:- (see note below) (a) Disclosable Pecuniary Interests (b) Private Interests (pecuniary and non-pecuniary) 4 Annual Update - Holme Pierrepont Country Park 7 - 8 5 Nottinghamshire and Nottingham Local Aggregates Assessment - 9 - 50 2017 Sales Data 6 Update on Key Trading Standards and Communities Matters 51 - 60 7 Cultural Services Events Programme 61 - 70 8 Future Highways Commissioning Arrangements 71 - 76 9 Highways Capital Programme 2019-2020 Additional Schemes 77 - 90 10 Nottinghamshire County Council (Queens Road North Eastwood) 91 - 100 (Prohibition of Waiting and Residents Controlled Zone) Traffic Regulation Order 2019 (5258) - Final Page 1 of 124 11 The Nottinghamshire County Council (Ashwell Street and Knight 101 - Street, Netherfield) (Prohibition of Waiting) Traffic Regulation Order 108 2019 (7204) 12 The Nottinghamshire County Council (Nordean and Somersby 109 - Road, Woodthorpe) (Prohibition of Parking Places) Traffic 118 Regulation Order 2019 (7206) 13 Work Programme 119 - 124 Notes (1) Councillors are advised to contact their Research Officer for details of any Group Meetings which are planned for this meeting. (2) Members of the public wishing to inspect "Background Papers" referred to in the reports on the agenda or Schedule 12A of the Local Government Act should contact:- Customer Services Centre 0300 500 80 80 (3) Persons making a declaration of interest should have regard to the Code of Conduct and the Council’s Procedure Rules. -

10/02/2021 MEMBERS INTERESTS S Page 1
MEMBERS INTERESTS S 11/09/2021 ID SURNAME PLACE NAME CODE DATES 4422 Sabine Hampshire HAM 1750 onwards 1058 Salisbury London LND c.19 5876 Salsbury Nottingham NTT 1830 - 1898 0320 Sanders Rotterdam pre 1877 0320 Sanders Holland LAN pre 1877 0691 Sanders Worcestershire WOR Any 1237 Sanderson Hose LEI pre 1700 5215 Sansom Nottingham NTT 1861+ 5215 Sansom Mansfield NTT 1600+ 0275 Sansom Nottingham NTT c.19 3685 Sargeant Scithon LIN 1800 3685 Sargeant Swineshead LIN 1800 3685 Sargent Swineshead LIN 1800 3685 Sargent Scothon LIN 1800 5113 Saunders Derbyshire DBY 1800 5113 Saunders Kirkby-in-Ashfield NTT 1800 5741 Saunders Worksop, Notts NTT Post 1900 2486 Savage Ashborne DBY pre 1920 0257 Saxton Greasley NTT pre 1800 1486 Saxton Papplewick NTT pre 1700 2919 Saxton Selston NTT pre 1850 2675 Sayers Lowdham NTT 1734-1739 2675 Sayers Hoveringham NTT 1742-1759 0275 Scales Newark NTT 1800-1900 0275 Scales Nottingham NTT 1800-1900 5182 Scarborough Bottesford LEI 1700 5899 Scarborough Newark NTT 1790's 5899 Scarborough Lincolnshire LIN 1790's 5182 Scarborough Harby LEI 1600s 3384 Scarborough Long Clawson LEI 1800 3323 Scargill Sheffield YKS c.19-c.20 3987 Scothern Woodborough NTT 1800 2985 Scott Sutton-in-Ashfield NTT Any 5938 Scott Scotland SCT Any 5938 Scott Lancashire LAN Any 5938 Scott Nottinghamshire NTT Any 1289 Scotthen Nottingham NTT c.18 0555 Scrimshaw Nottinghamshire NTT 1750 5642 Scrimshaw Cotgrave NTT 1700 - 1900 5642 Scrimshaw Radcliffe-on-Trent NTT 1700 - 1900 5642 Scrimshire Cotgrave NTT 1600 - 1800 5642 Scrimshire Radcliffe-on-Trent -

Aligned Core Strategy
Greater Nottingham Broxtowe Borough Gedling Borough Nottingham City Aligned Core Strategies Part 1 Local Plan Adopted September 2014 Contact Details: Broxtowe Borough Council Foster Avenue Beeston Nottingham NG9 1AB Tel: 0115 9177777 [email protected] www.broxtowe.gov.uk/corestrategy Gedling Borough Council Civic Centre Arnot Hill Park Arnold Nottingham NG5 6LU Tel: 0115 901 3757 [email protected] www.gedling.gov.uk/gedlingcorestrategy Nottingham City Council LHBOX52 Planning Policy Team Loxley House Station Street Nottingham NG2 3NG Tel: 0115 876 3973 [email protected] www.nottinghamcity.gov.uk/corestrategy General queries about the process can also be made to: Greater Nottingham Growth Point Team Loxley House Station Street Nottingham NG2 3NG Tel 0115 876 2561 [email protected] www.gngrowthpoint.com Alternative Formats All documentation can be made available in alternative formats or languages on request. Contents Working in Partnership to Plan for Greater Nottingham 1 1.1 Working in Partnership to Plan for Greater Nottingham 1 1.2 Why the Councils are Working Together 6 1.3 The Local Plan (formerly Local Development Framework) 6 1.4 Sustainability Appraisal 9 1.5 Habitats Regulations Assessment 10 1.6 Equality Impact Assessment 11 The Future of Broxtowe, Gedling and Nottingham City in the Context of Greater Nottingham 13 2.1 Key Influences on the Future of the Plan Area 13 2.2 The Character of the Plan Area 13 2.3 Spatial Vision 18 2.4 Spatial Objectives 20 2.5 Links to Sustainable Community