Environmental Engineering Lab Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Design and Test of a Miniature Hydrogen Production Integrated Reactor
reactions Article Design and Test of a Miniature Hydrogen Production Integrated Reactor Ion Velasco, Oihane Sanz * , Iñigo Pérez-Miqueo, Iñigo Legorburu and Mario Montes Department Applied Chemistry, University of the Basque Country (UPV/EHU), 20018 San Sebastian, Spain; [email protected] (I.V.); [email protected] (I.P.-M.); [email protected] (I.L.); [email protected] (M.M.) * Correspondence: [email protected] Abstract: A detailed study of the experimental issues involved in the design and operation of a methanol steam microreformer is presented in this paper. Micromachining technology was utilized to fabricate a metallic microchannel block coupling the exothermic and endothermic process. The microchannel block was coated with a Pd/ZnO catalyst in the reforming channels and with Pd/Al2O3 in the combustion channels by washcoating. An experimental system had been designed and fine- tuned allowing estimation of the heat losses of the system and to compensate for them by means of electric heating cartridges. In this way, the heat necessary for the reforming reaction is provided by methanol combustion, thanks to the temperature and flow cascade controller we developed. Thus, the coupling of both reactions in a block of microchannels without the interference caused by significant heat loss due to the small size of the laboratory microreactor could be studied. Runs of this microreformer device were carried out, varying the deposited catalyst amount, methanol steam Citation: Velasco, I.; Sanz, O.; reforming temperature and space velocity. When the reforming reaction was compensated by the Pérez-Miqueo, I.; Legorburu, I.; combustion reaction and the heat losses by the electric heating, an almost isothermal behavior of the Montes, M. -

INTERMEDIATE LABORATORY - PHYX 3870-3880 List of Experiments Fall 2015 MECHANICS M1
INTERMEDIATE LABORATORY - PHYX 3870-3880 List of Experiments Fall 2015 MECHANICS M1. Kater's Pendulum A straightforward lab using a reversible physical pendulum to measure the local acceleration of gravity, g, to less than 0.01%. Emphasis is given to detailed error analysis and error propagation and to high precision measurements. Computer interfacing with photogates and motion sensors. A good beginning lab. M2. Coupled Pendulum Investigation of two pendulums coupled by a spring between them. Lab emphasizes comparison of measured results of the periods and damping to values derived from a theoretical model using differential equations. Completion of Analytical Mechanics is recommended. Computerized data collection with voltage probes and motion sensors and data reduction are used. ELECTRICITY & MAGNETISM E2. Thompson's e/m Experiment Measures the ratio of the charge to mass of an electron (e/m) by investigating the trajectory of electrons in electric and magnetic fields. The British physicist J. J. Thompson received a Nobel prize for the experiment. Good preparation for Electron Diffraction from a Crystal Lattice and subsequent Advanced Laboratory experiments. Emphasis is on determining the interdependence of experimental parameters (e.g., accelerating voltage, magnetic field, radius, charge) through the Lorentz Force Law. A good beginning lab. THERMODYNAMICS/STATISTICAL MECHANICS T1. Velocity and Gravitational Distributions An excellent introduction to the kinetic theory of gases. Uses an air table to investigate the statistical distribution of the velocity of particles. Compares the results with Statistical Mechanics velocity distribution functions. Also explores the effect of an external field (gravity) on the statistical distribution of particles. Emphasizes statistical modeling. Computer interfacing uses a video camera. -

Elegant Solutions
Book Reviews 157 THE END OF SILENT RITES the top ten list, you might respond. The list includes, of course, the most famous Philip Ball: Elegant Solutions: Ten heroes from the history of chemistry Beautiful Experiments in Chemistry , and captures their most important The Royal Society of Chemistry, breakthrough experiments. By copying Cambridge, UK, 2005, vii+212 pp. or transferring to current research issues [ISBN 0-85404-674-7] the heroic deeds of the past, young scholars can accomplish excellence. In 2002 the American Chemical Society Now, if the key to doing important (ACS) asked its members to submit chemistry is learning from the history of proposals for the “ten most beautiful chemistry, why did the ACS encourage experiments in chemistry” ( C&EN , doing history of chemistry in the clum- Nov. 18, 2002, p. 5) and then proudly siest manner one can imagine – by col- published the result of the vote in its lecting and ranking decontextualized Chemical and Engineering News maga- historical ‘facts’ and anecdotes from the zine ( C&EN , Aug. 25, 2003, pp. 27-30). memory of its members who used to Democratic as the procedure is, it avoids have no training in the history of chem- asking critical questions: What is an ex- istry? Because the scholarly historiog- periment? What is beauty? What is raphy of chemistry does not matter chemistry? In fact, you need not be able here, you might respond. What matters to give an answer to these questions in is only what today’s chemists consider order to vote. We could even imagine to be important chemistry of the past, none of the voters being able to answer be that invented anecdotes or not. -
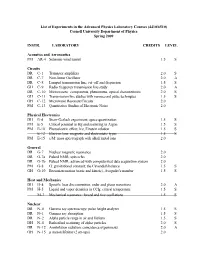
List of Experiments in the Advanced Physics Laboratory Courses (4410/6510) Cornell University Department of Physics Spring 2009
List of Experiments in the Advanced Physics Laboratory Courses (4410/6510) Cornell University Department of Physics Spring 2009 INSTR. LABORATORY CREDITS LEVEL Acoustics and Aeronautics PM AR-4 Subsonic wind tunnel 1.5 S Circuits DR C-1 Transistor amplifiers 2.0 S DR C-7 Non-linear Oscillator 2.0 A DR C-8 Lumped transmission line; cut-off and dispersion 1.5 S GH C-9 Radio frequency transmission line study 2.0 A DR C-10 Microwaves: components, phenomena, optical characteristics 2.0 S GH C-11 Transmission line studies with nanosecond pulse techniques 1.5 S GH C-12 Microwave Resonant Circuits 2.0 PM C-13 Quantitative Studies of Electronic Noise 2.0 Physical Electronics DH E-4 Stern-Gerlach experiment, space quantization 1.5 S PM E-5 Critical potential in Hg and scattering in Argon 1.5 S PM E-10 Photoelectric effect; h/e, Einstein relation 1.5 S E-12 Electron lens: magnetic and electrostatic types 1.5 S PM E-15 e/M: mass spectrograph with alkali metal ions 2.0 General DR G-7 Nuclear magnetic resonance 2.0 DR G-7a Pulsed NMR; spin echo 2.0 DR G-7b Pulsed NMR; advanced with computerized data acquisition system 2.0 PM G-8 G: gravitational constant; the Cavendish balance 1.5 S GH G-10 Brownian motion (static and kinetic), Avogadro's number 1.5 S Heat and Mechanics DH H-4 Specific heat discontinuities; order and phase transitions 2.0 A PM H-5 Liquid and vapor densities in CCl4; critical temperature 1.5 S M-3 Mechanical resonance: forced and free oscillations 1.5 S Nuclear DR N-0 Gamma ray spectroscopy: pulse height analyzer 1.5 S DR N-1 Gamma -

The Captive Lab Rat: Human Medical Experimentation in the Carceral State
Boston College Law Review Volume 61 Issue 1 Article 2 1-29-2020 The Captive Lab Rat: Human Medical Experimentation in the Carceral State Laura I. Appleman Willamette University, [email protected] Follow this and additional works at: https://lawdigitalcommons.bc.edu/bclr Part of the Bioethics and Medical Ethics Commons, Criminal Law Commons, Disability Law Commons, Health Law and Policy Commons, Juvenile Law Commons, Law and Economics Commons, Law and Society Commons, Legal History Commons, and the Medical Jurisprudence Commons Recommended Citation Laura I. Appleman, The Captive Lab Rat: Human Medical Experimentation in the Carceral State, 61 B.C.L. Rev. 1 (2020), https://lawdigitalcommons.bc.edu/bclr/vol61/iss1/2 This Article is brought to you for free and open access by the Law Journals at Digital Commons @ Boston College Law School. It has been accepted for inclusion in Boston College Law Review by an authorized editor of Digital Commons @ Boston College Law School. For more information, please contact [email protected]. THE CAPTIVE LAB RAT: HUMAN MEDICAL EXPERIMENTATION IN THE CARCERAL STATE LAURA I APPLEMAN INTRODUCTION ................................................................................................................................ 2 I. A HISTORY OF CAPTIVITY AND EXPERIMENTATION .................................................................... 4 A. Asylums and Institutions ........................................................................................................ 5 B. Orphanages, Foundling -

INTERMEDIATE LABORATORY - PHYX 3870-3880 Description of Experiments
INTERMEDIATE LABORATORY - PHYX 3870-3880 Description of Experiments MECHANICS M1. Kater's Pendulum A straightforward lab using a reversible physical pendulum to measure the local acceleration of gravity, g, to less than 0.01%. Emphasis is given to detailed error analysis and error propagation and to high precision measurements. Computer interfacing with photogates and motion sensors. A good beginning lab. M2. Coupled Pendulum Investigation of two pendulums coupled by a spring between them. Lab emphasizes comparison of measured results of the periods and damping to values derived from a theoretical model using differential equations. Completion of Analytical Mechanics is recommended. Computerized data collection with voltage probes and motion sensors and data reduction are used. M3. Cavendish Balance Determines the universal gravitational constant, G, using a torsion balance to measure the attraction between known masses. Emphasis is on fitting the data with a detailed model and extracting results by correlating the fitting parameters with the physical model. A good beginning lab. ELECTRICITY & MAGNETISM E1. Millikan's Oil Drop Experiment Demonstrates the quantized nature of the electric charge and measures a value of the fundamental constant e. This classic experiment led to the first Nobel prize for an American physicist. Emphasizes experimental design and statistical analysis methods using large data sets. E2. Thompson's e/m Experiment Measures the ratio of the charge to mass of an electron (e/m) by investigating the trajectory of electrons in electric and magnetic fields. The British physicist J. J. Thompson received a Nobel prize for the experiment. Good preparation for Electron Diffraction from a Crystal Lattice and subsequent Advanced Laboratory experiments. -

Josiah Parsons Cooke
MEMOIR JOSIAH PARSONS COOKE. Q97 i CHARLES L. JACKSON. PREPARED FOR THE NATIONAL ACADEMY. (26) 175 BIOGRAPHICAL MEMOIR OF JOSIAH PARSONS COOKE. JOSIAH PARSONS COOKE, the son of Josiah Parsons Cooke, a successful lawyer, and Mary (Pratt) Cooke, was born in Boston October 12,1827. He was educated at the Boston Latin School and Harvard College, from which he graduated in 1848, and after a year of travel in Europe was appointed tutor in math- ematics in Harvard College in 1849. In 1850 he became Erving Professor of Chemistry and Mineralogy, a position which he held for the remainder of his life. Cooke's equipment for the duties of his new place was almost entirely the result of his own exertions. A course of lectures by the elder Silliman first aroused his enthusiasm for chemistry and led him in early boyhood to fit up a laboratory in his father's house, where he attacked the science by experiment with such good results that even when he came to college he had a work- ing knowledge of the subject. At Cambridge he continued these studies essentially alone, as the chemical teaching of the college during his four 3'ears of residence was confined to five or six rather disjointed and fragmentary lectures. Immediately after appointment to his professorship he supplemented these meager preparations by obtaining a leave of absence for eight months, which were spent in Europe buying apparatus and material and attending lectures by Regnault and Dumas. These formed the only instruction in chemistry he had received which could even claim to be systematic; yet with this slender outfit, aided by barely a year and a half of experience as a teacher, in 1851, at the age of 24, he found himself confronted with problems which would have taxed the abilities of an old, experienced, and fully educated professor. -

Chapter 9: Enabling Capabilities for Science and Energy September 2015 9 Enabling Capabilities for Science and Energy
QUADRENNIAL TECHNOLOGY REVIEW AN ASSESSMENT OF ENERGY TECHNOLOGIES AND RESEARCH OPPORTUNITIES Chapter 9: Enabling Capabilities for Science and Energy September 2015 9 Enabling Capabilities for Science and Energy Tools for Scientific Discovery and Technology Development Investment in basic science research is expanding our understanding of how structure leads to function—from the atomic- and nanoscale to the mesoscale and beyond—in natural systems, and is enabling a transformation from observation to control and design of new systems with properties tailored to meet the requirements of the next generation of energy technologies. At the core of this new paradigm is a suite of experimental and computational tools that enable researchers to probe and manipulate matter at unprecedented resolution. The planning and development of these tools is rooted in basic science, but they are critically important for technology development, enabling discoveries that can lead to broad implementation. These tools are available through a user facility model that provides merit-based open access for nonproprietary research. Each year, thousands of users leverage the capabilities and staff expertise for their research, while the facilities leverage user expertise toward maintenance, development, and application of the tools in support of the broader community of users. The challenges in energy science and technology development increasingly necessitate interdisciplinary collaboration. The multidisciplinary and multi-institutional research centers supported by DOE are designed to integrate basic science and applied research to accelerate development of new and transformative energy technologies. Capabilities supported by DOE and the DOE-Office of Science (SC) are enabling the following across science and technology: - The X-ray light and neutron sources provide unprecedented access to the structure and dynamics of materials and the molecular-scale basis of chemical reactions. -

Computer Science and Engineering
Syllabus of UNDERGRADUATE DEGREE COURSE B.Tech. V Semester Computer Science and Engineering Rajasthan Technical University, Kota Effective from session: 2019 – 2020 RAJASTHAN TECHNICAL UNIVERSITY, KOTA Syllabus III Year-V Semester: B.Tech. Computer Science and Engineering 5CS3-01: Information Theory & Coding Credit: 2 Max. Marks: 100(IA:20, ETE:80) 2L+0T+0P End Term Exam: 2 Hours SN Contents Hours 1 Introduction:Objective, scope and outcome of the course. 01 2 Introduction to information theory: Uncertainty, Information and Entropy, Information measures for continuous random 05 variables, source coding theorem. Discrete Memory less channels, Mutual information, Conditional entropy. 3 Source coding schemes for data compaction: Prefix code, Huffman code, Shanon-Fane code &Hempel-Ziv coding channel 05 capacity. Channel coding theorem. Shannon limit. 4 Linear Block Code: Introduction to error connecting codes, coding & decoding of linear block code, minimum distance consideration, 05 conversion of non-systematic form of matrices into systematic form. 5 Cyclic Code: Code Algebra, Basic properties of Galois fields (GF) polynomial operations over Galois fields, generating cyclic code by generating polynomial, parity check polynomial. Encoder & 06 decoder for cyclic codes. 6 Convolutional Code: Convolutional encoders of different rates. Code Tree, Trllis and state diagram. Maximum likelihood decoding 06 of convolutional code: The viterbi Algorithm fee distance of a convolutional code. Total 28 Syllabus of 3rdYear B. Tech. (CS) for students admitted in Session 2017-18 onwards. Page 2 RAJASTHAN TECHNICAL UNIVERSITY, KOTA Syllabus III Year-V Semester: B.Tech. Computer Science and Engineering 5CS4-02: Compiler Design Credit: 3 Max. Marks: 150(IA:30, ETE:120) 3L+0T+0P End Term Exam: 3 Hours SN Contents Hours 1 Introduction:Objective, scope and outcome of the course. -

“Energy, Electricity and Hydrogen” Thesaurus of Suggested Didactical Experiments
“Energy, Electricity and Hydrogen” Thesaurus of suggested didactical experiments Structure of experiments The proposed experiments constitute a list, from which teachers, according to their local educational needs/ possibilities may construct own scenarios. We suggest that chosen elements from this list are inserted into the preferred by teachers lesson scenarios. Therefore, the list does not contain “must-have” experiments, but an open directory. By the color we sign the level of difficulty and/or complexity and/or special safety requirements: green – simple (in our intent possible to experiment in the age 8-10 yrs), blue – intermediate (11-12 yrs), red – difficult (13-17 yrs), violet – expert (not only difficult, but principally expensive). Main contents We start from very simple experiments on mechanical energy that can be done on the elementary level. Then we discuss experiment in electrochemistry and electromagnetism (4 experiments on electrochemistry on elementary-school level are described separately, with worksheets). Alternative energies and fuel cell are discussed in details in separate documents, on the secondary level The experiments are organized to follow the “production” line of energy making a fuel-cell car move. LIST OF EXPERIMENTS 1. Energy, its forms and energy conversion These experiments aim to show that the energy cannot be “produced” but only transformed from one type to another: mechanical energy, electricity, heat. ● 1.1. Jumping balls: a heavier rubber ball (make a small cavity in the upper side) and a ping- pong ball. This is the simplest experiment that (from our didactical experience with children) spontaneously induces the idea of energy: children exclaim “the heavier ball transmitted the energy to the upper one”. -
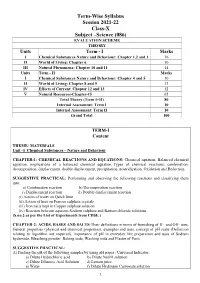
Term-Wise Syllabus Session 2021-22 Class-X Subject –Science
Term-Wise Syllabus Session 2021-22 Class-X Subject –Science (086) EVALUATION SCHEME THEORY Units Term - I Marks I Chemical Substances-Nature and Behaviour: Chapter 1,2 and 3 16 II World of Living: Chapter 6 10 III Natural Phenomena: Chapter 10 and 11 14 Units Term - II Marks I Chemical Substances-Nature and Behaviour: Chapter 4 and 5 10 II World of Living: Chapter 8 and 9 13 IV Effects of Current: Chapter 12 and 13 12 V Natural Resources-Chapter-15 05 Total Theory (Term I+II) 80 Internal Assessment: Term I 10 Internal Assessment: Term II 10 Grand Total 100 TERM-I Content THEME: MATERIALS Unit –I Chemical Substances – Nature and Behaviour CHAPTER-1: CHEMICAL REACTIONS AND EQUATIONS- Chemical equation, Balanced chemical equation, implications of a balanced chemical equation, Types of chemical reactions: combination, decomposition, displacement, double displacement, precipitation, neutralization, Oxidation and Reduction. SUGGESTIVE PRACTICAL: Performing and observing the following reactions and classifying them into: a) Combination reaction b) Decomposition reaction c) Displacement reaction d) Double displacement reaction (i) Action of water on Quick lime (ii) Action of heat on Ferrous sulphate crystals (iii) Iron nails kept in Copper sulphate solution (iv) Reaction between aqueous Sodium sulphate and Barium chloride solutions (S.no.2 as per the List of Experiments from CBSE.) CHAPTER-2: ACIDS, BASES AND SALTS-Their definitions in terms of furnishing of H+ and OH- ions, General properties (physical and chemical properties), examples and uses, concept of pH scale (Definition relating to logarithm not required), importance of pH in everyday life; preparation and uses of Sodium hydroxide, Bleaching powder, Baking soda, Washing soda and Plaster of Paris. -

Physics 480W, Experimental Modern Physics
Physics 480W, Experimental Modern Physics Dr. Greg Severn MWF 1:25-2:20 ST252, ST285 (Lectures & Tutorials) T 2:30-5:20 ST290 & ST 287 (Laboratory experiments) (Dated: Spring 2018 Draft Version 1.0) Professor: Dr. Greg Severn, ST285 x6845, sev- physics and plasma physics. Attention to the practical [email protected] side of testing advanced modern physical theories in the laboratory is an important part of the course, as Office Hours: MW 1:30-3:50, T 10-11:00, & by appoint- is grasping the integration and interplay of theory and ment. Appointments are always possible. experiment. Coming to understand how theory and Catalog listing: EXPERIMENTAL MODERN PHYSICS experiment relate to each other in carefully designed Units: 4, Prerequisites: PHYS 330 (Quantum experiments is an essential part of understanding how Mechanics), A laboratory-based course science advances. And for all these reasons, this course focused on the introduction to principles is a good introduction to the world of physics research. of research techniques with an emphasis Mastery of experimental and theoretical ideas will be on modern physics. Experiments illustrate demonstrated through well written summary papers. physical phenomena pertaining to core areas These will conform to current standards for research of physics: quantum mechanics, atomic and papers in Physics, and this writing intensive course is nuclear physics, laser physics and plasma the first course in our curriculum in which this skill physics. Analog and digital data acquisition is inculcated. Above all, the student is introduced instrumentation, high-resolution optical to the review process to which all contribu- and laser technology, and phase sensitive tions to the scientific literature (worthy of the detection technology will be explored.