The Captive Lab Rat: Human Medical Experimentation in the Carceral State
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Hidden Structure of Overimitation
The hidden structure of overimitation Derek E. Lyons*†, Andrew G. Young‡, and Frank C. Keil* *Department of Psychology, Yale University, Box 208205, New Haven, CT 06520; and ‡Department of Psychology, University of Wisconsin, Brogden Hall, Madison, WI 53706 Edited by Susan E. Carey, Harvard University, Cambridge, MA, and approved October 18, 2007 (received for review May 11, 2007) Young children are surprisingly judicious imitators, but there are than in the utility of the actions that they copy. Others suggest that also times when their reproduction of others’ actions appears children overimitate ‘‘because they [see] the behavior of the dem- strikingly illogical. For example, children who observe an adult onstrator as intentional, even if they did appreciate that some parts inefficiently operating a novel object frequently engage in what of the demonstration were causally irrelevant’’ (ref. 10, p. 179). That we term overimitation, persistently reproducing the adult’s un- is, the intentionality of the adult’s action may constitute an implicit necessary actions. Although children readily overimitate irrelevant social demand for children, leading them to infer that they are actions that even chimpanzees ignore, this curious effect has ‘‘supposed’’ to imitate. A final possibility is that overimitation may previously attracted little interest; it has been assumed that chil- simply be a byproduct of habit. Overimitation may arise, in other dren overimitate not for theoretically significant reasons, but words, because imitation ‘‘remains habitual even in a specific rather as a purely social exercise. In this paper, however, we situation in which less fidelity would actually afford more effi- challenge this view, presenting evidence that overimitation re- ciency’’ (ref. -

Design and Test of a Miniature Hydrogen Production Integrated Reactor
reactions Article Design and Test of a Miniature Hydrogen Production Integrated Reactor Ion Velasco, Oihane Sanz * , Iñigo Pérez-Miqueo, Iñigo Legorburu and Mario Montes Department Applied Chemistry, University of the Basque Country (UPV/EHU), 20018 San Sebastian, Spain; [email protected] (I.V.); [email protected] (I.P.-M.); [email protected] (I.L.); [email protected] (M.M.) * Correspondence: [email protected] Abstract: A detailed study of the experimental issues involved in the design and operation of a methanol steam microreformer is presented in this paper. Micromachining technology was utilized to fabricate a metallic microchannel block coupling the exothermic and endothermic process. The microchannel block was coated with a Pd/ZnO catalyst in the reforming channels and with Pd/Al2O3 in the combustion channels by washcoating. An experimental system had been designed and fine- tuned allowing estimation of the heat losses of the system and to compensate for them by means of electric heating cartridges. In this way, the heat necessary for the reforming reaction is provided by methanol combustion, thanks to the temperature and flow cascade controller we developed. Thus, the coupling of both reactions in a block of microchannels without the interference caused by significant heat loss due to the small size of the laboratory microreactor could be studied. Runs of this microreformer device were carried out, varying the deposited catalyst amount, methanol steam Citation: Velasco, I.; Sanz, O.; reforming temperature and space velocity. When the reforming reaction was compensated by the Pérez-Miqueo, I.; Legorburu, I.; combustion reaction and the heat losses by the electric heating, an almost isothermal behavior of the Montes, M. -

Andrea Deoudes, Kinetics: a Clock Reaction
A Kinetics Experiment The Rate of a Chemical Reaction: A Clock Reaction Andrea Deoudes February 2, 2010 Introduction: The rates of chemical reactions and the ability to control those rates are crucial aspects of life. Chemical kinetics is the study of the rates at which chemical reactions occur, the factors that affect the speed of reactions, and the mechanisms by which reactions proceed. The reaction rate depends on the reactants, the concentrations of the reactants, the temperature at which the reaction takes place, and any catalysts or inhibitors that affect the reaction. If a chemical reaction has a fast rate, a large portion of the molecules react to form products in a given time period. If a chemical reaction has a slow rate, a small portion of molecules react to form products in a given time period. This experiment studied the kinetics of a reaction between an iodide ion (I-1) and a -2 -1 -2 -2 peroxydisulfate ion (S2O8 ) in the first reaction: 2I + S2O8 I2 + 2SO4 . This is a relatively slow reaction. The reaction rate is dependent on the concentrations of the reactants, following -1 m -2 n the rate law: Rate = k[I ] [S2O8 ] . In order to study the kinetics of this reaction, or any reaction, there must be an experimental way to measure the concentration of at least one of the reactants or products as a function of time. -2 -2 -1 This was done in this experiment using a second reaction, 2S2O3 + I2 S4O6 + 2I , which occurred simultaneously with the reaction under investigation. Adding starch to the mixture -2 allowed the S2O3 of the second reaction to act as a built in “clock;” the mixture turned blue -2 -2 when all of the S2O3 had been consumed. -

Introduction
Introduction The first volume of Edward Strutt Abdy’s Journal of a Residence and Tour in the United States of North America (1835) contains a surprising anecdote about the practice of science by African Americans in the early nineteenth century. Abdy was part of a delegation sent by the English government to tour American prisons. His journal intersperses accounts of the prison system with a narrative of his travels, especially as they reflected the author’s observations about race and slavery in the United States. During an account of his time in New York City, Abdy writes about his visit to St. Philip’s Episcopal Church in Lower Manhattan, whose members included many of the most prominent black families in the city.1 Given the popular dissemination of ethnographic accounts of slavery in transatlantic travelogues and newspapers, British readers would surely be interested to hear details about an Episcopal church run by free African Americans in the US North. But before taking readers into the pews of St. Philip’s to hear a sermon by the Reverend Peter Wil- liams, Abdy dwells in the graveyard that belonged to the congregation on Chrystie Street, taking a surprising detour from black theology to black craniology. There in the graveyard, Abdy recounts, he had a conversation with St. Philip’s sexton, the warden of the church’s cemetery and its head grave- digger. He is keen to relate the sexton’s own observations about the re- mains under his stewardship, especially his claim that the skulls that had been buried for many years in his graveyard—those, -

INTERMEDIATE LABORATORY - PHYX 3870-3880 List of Experiments Fall 2015 MECHANICS M1
INTERMEDIATE LABORATORY - PHYX 3870-3880 List of Experiments Fall 2015 MECHANICS M1. Kater's Pendulum A straightforward lab using a reversible physical pendulum to measure the local acceleration of gravity, g, to less than 0.01%. Emphasis is given to detailed error analysis and error propagation and to high precision measurements. Computer interfacing with photogates and motion sensors. A good beginning lab. M2. Coupled Pendulum Investigation of two pendulums coupled by a spring between them. Lab emphasizes comparison of measured results of the periods and damping to values derived from a theoretical model using differential equations. Completion of Analytical Mechanics is recommended. Computerized data collection with voltage probes and motion sensors and data reduction are used. ELECTRICITY & MAGNETISM E2. Thompson's e/m Experiment Measures the ratio of the charge to mass of an electron (e/m) by investigating the trajectory of electrons in electric and magnetic fields. The British physicist J. J. Thompson received a Nobel prize for the experiment. Good preparation for Electron Diffraction from a Crystal Lattice and subsequent Advanced Laboratory experiments. Emphasis is on determining the interdependence of experimental parameters (e.g., accelerating voltage, magnetic field, radius, charge) through the Lorentz Force Law. A good beginning lab. THERMODYNAMICS/STATISTICAL MECHANICS T1. Velocity and Gravitational Distributions An excellent introduction to the kinetic theory of gases. Uses an air table to investigate the statistical distribution of the velocity of particles. Compares the results with Statistical Mechanics velocity distribution functions. Also explores the effect of an external field (gravity) on the statistical distribution of particles. Emphasizes statistical modeling. Computer interfacing uses a video camera. -
![Philosophia Scientiæ, 19-3 | 2015, « the Bounds of Naturalism » [Online], Online Since 03 November 2017, Connection on 10 November 2020](https://docslib.b-cdn.net/cover/5884/philosophia-scienti%C3%A6-19-3-2015-%C2%AB-the-bounds-of-naturalism-%C2%BB-online-online-since-03-november-2017-connection-on-10-november-2020-505884.webp)
Philosophia Scientiæ, 19-3 | 2015, « the Bounds of Naturalism » [Online], Online Since 03 November 2017, Connection on 10 November 2020
Philosophia Scientiæ Travaux d'histoire et de philosophie des sciences 19-3 | 2015 The Bounds of Naturalism Experimental Constraints and Phenomenological Requiredness Charles-Édouard Niveleau and Alexandre Métraux (dir.) Electronic version URL: http://journals.openedition.org/philosophiascientiae/1121 DOI: 10.4000/philosophiascientiae.1121 ISSN: 1775-4283 Publisher Éditions Kimé Printed version Date of publication: 30 October 2015 ISBN: 978-2-84174-727-6 ISSN: 1281-2463 Electronic reference Charles-Édouard Niveleau and Alexandre Métraux (dir.), Philosophia Scientiæ, 19-3 | 2015, « The Bounds of Naturalism » [Online], Online since 03 November 2017, connection on 10 November 2020. URL : http://journals.openedition.org/philosophiascientiae/1121 ; DOI : https://doi.org/10.4000/ philosophiascientiae.1121 This text was automatically generated on 10 November 2020. Tous droits réservés 1 TABLE OF CONTENTS The Bounds of Naturalism: A Plea for Modesty Charles-Édouard Niveleau and Alexandre Métraux A Philosopher in the Lab. Carl Stumpf on Philosophy and Experimental Sciences Riccardo Martinelli La phénoménologie expérimentale d’Albert Michotte : un problème de traduction Sigrid Leyssen Objectifying the Phenomenal in Experimental Psychology: Titchener and Beyond Gary Hatfield Spatial Elements in Visual Awareness. Challenges for an Intrinsic “Geometry” of the Visible Liliana Albertazzi The Phenomenology of the Invisible: From Visual Syntax to “Shape from Shapes” Baingio Pinna, Jan Koenderink and Andrea van Doorn The Deep Structure of Lives Michael Kubovy Philosophia Scientiæ, 19-3 | 2015 2 The Bounds of Naturalism: A Plea for Modesty Charles-Édouard Niveleau and Alexandre Métraux Many thanks to William Blythe for his insightful linguistic corrections on the English text. 1 Introduction 1 The articles published in this volume of Philosophia Scientiæ address specific issues in contemporary psychological research. -

Elegant Solutions
Book Reviews 157 THE END OF SILENT RITES the top ten list, you might respond. The list includes, of course, the most famous Philip Ball: Elegant Solutions: Ten heroes from the history of chemistry Beautiful Experiments in Chemistry , and captures their most important The Royal Society of Chemistry, breakthrough experiments. By copying Cambridge, UK, 2005, vii+212 pp. or transferring to current research issues [ISBN 0-85404-674-7] the heroic deeds of the past, young scholars can accomplish excellence. In 2002 the American Chemical Society Now, if the key to doing important (ACS) asked its members to submit chemistry is learning from the history of proposals for the “ten most beautiful chemistry, why did the ACS encourage experiments in chemistry” ( C&EN , doing history of chemistry in the clum- Nov. 18, 2002, p. 5) and then proudly siest manner one can imagine – by col- published the result of the vote in its lecting and ranking decontextualized Chemical and Engineering News maga- historical ‘facts’ and anecdotes from the zine ( C&EN , Aug. 25, 2003, pp. 27-30). memory of its members who used to Democratic as the procedure is, it avoids have no training in the history of chem- asking critical questions: What is an ex- istry? Because the scholarly historiog- periment? What is beauty? What is raphy of chemistry does not matter chemistry? In fact, you need not be able here, you might respond. What matters to give an answer to these questions in is only what today’s chemists consider order to vote. We could even imagine to be important chemistry of the past, none of the voters being able to answer be that invented anecdotes or not. -

Duck and Cover: How Print Media, the U.S. Government, and Entertainment Culture Formedamerica's Understanding of the Atom
DUCK AND COVER: HOW PRINT MEDIA, THE U.S. GOVERNMENT, AND ENTERTAINMENT CULTURE FORMEDAMERICA’S UNDERSTANDING OF THE ATOM BOMB A thesis submitted in partial fulfillment of the requirements for the degree of Master of Arts By Daniel Patrick Wright B.A., University of Cincinnati, 2013 2015 Wright State University WRIGHT STATE UNIVERSITY GRADUATE SCHOOL May 5, 2015 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Daniel Patrick Wright ENTITLED Duck and Cover: How Print Media, the U.S. Government and Entertainment Culture Formed America’s Understanding of the Atom Bomb BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Arts ________________________________ Jonathan Winkler, Thesis Director ________________________________ Carol Herringer, Chair History Department Committee on College of Liberal Arts Final Examination ________________________________ Drew Swanson, Ph.D. ________________________________ Nancy Garner, Ph.D. ________________________________ Robert E. W. Fyffe, Ph.D. Vice President for Research and Dean of the Graduate School ABSTRACT Wright, Daniel Patrick. M.A. Department of History, Wright State University, 2015. Duck and Cover: How Print Media, the U.S. Government and Entertainment Culture Formed America’s Understanding of the Atom Bomb This research project will explore an overview of the different subsections of American post-war society that contributed to the American “atomic reality” in hopes of revealing how and why the American understanding of atomic weapons did not slowly evolve over the course of a generation, but instead materialize rapidly in the years following the bombing of Hiroshima and Nagasaki. By analyzing government sources and programs, print media sources such as newspapers and magazines, and the American entertainment culture of the 1940s and 1950s, this research project will answer exactly why and how the American public arrived at its understanding of the atom bomb. -

Care and Custody in a Pennsylvania Prison
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2016 Wards Of The State: Care And Custody In A Pennsylvania Prison Nicholas Iacobelli University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Public Health Education and Promotion Commons, and the Social and Cultural Anthropology Commons Recommended Citation Iacobelli, Nicholas, "Wards Of The State: Care And Custody In A Pennsylvania Prison" (2016). Publicly Accessible Penn Dissertations. 2350. https://repository.upenn.edu/edissertations/2350 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/2350 For more information, please contact [email protected]. Wards Of The State: Care And Custody In A Pennsylvania Prison Abstract In this dissertation, I examine the challenges and contradictions as well as the expectations and aspirations involved in the provision of healthcare to inmates in a maximum-security prison in Pennsylvania. In 1976, the Supreme Court granted inmates a constitutional right to healthcare based on the notion that a failure to do so would constitute “cruel and unusual punishment.” Drawing on two years of ethnographic fieldwork from 2014-2016 in the prison’s medical unit with inmates, healthcare providers, and correctional staff, I demonstrate how the legal infrastructure built around this right to healthcare operates in practice and the myriad effects it has for those in state custody. Through traversing the scales of legal doctrine, privatized managed care, and collective historical memory, bringing these structural components to life in personal narratives and clinical interactions, I advance the notion that the physical space of the prison’s medical unit is a “ward of the state” – a space of care where the state itself is “made” through interactions among individuals who relay and enact the legal regulations on inmate healthcare. -

Science Fiction Stories with Good Astronomy & Physics
Science Fiction Stories with Good Astronomy & Physics: A Topical Index Compiled by Andrew Fraknoi (U. of San Francisco, Fromm Institute) Version 7 (2019) © copyright 2019 by Andrew Fraknoi. All rights reserved. Permission to use for any non-profit educational purpose, such as distribution in a classroom, is hereby granted. For any other use, please contact the author. (e-mail: fraknoi {at} fhda {dot} edu) This is a selective list of some short stories and novels that use reasonably accurate science and can be used for teaching or reinforcing astronomy or physics concepts. The titles of short stories are given in quotation marks; only short stories that have been published in book form or are available free on the Web are included. While one book source is given for each short story, note that some of the stories can be found in other collections as well. (See the Internet Speculative Fiction Database, cited at the end, for an easy way to find all the places a particular story has been published.) The author welcomes suggestions for additions to this list, especially if your favorite story with good science is left out. Gregory Benford Octavia Butler Geoff Landis J. Craig Wheeler TOPICS COVERED: Anti-matter Light & Radiation Solar System Archaeoastronomy Mars Space Flight Asteroids Mercury Space Travel Astronomers Meteorites Star Clusters Black Holes Moon Stars Comets Neptune Sun Cosmology Neutrinos Supernovae Dark Matter Neutron Stars Telescopes Exoplanets Physics, Particle Thermodynamics Galaxies Pluto Time Galaxy, The Quantum Mechanics Uranus Gravitational Lenses Quasars Venus Impacts Relativity, Special Interstellar Matter Saturn (and its Moons) Story Collections Jupiter (and its Moons) Science (in general) Life Elsewhere SETI Useful Websites 1 Anti-matter Davies, Paul Fireball. -

Acres of Skin
J7ournal ofMedical Ethics 1999;25:353-358 J Med Ethics: first published as 10.1136/jme.25.4.353 on 1 August 1999. Downloaded from Book reviews Acres of Skin Nazi reference is convenient but slip- be judged inadequate. In general the shod. At the centre of Nazi transgres- prisoners were so attracted by the sion was a public policy-enforced by compensation that, after twenty years Allen M Hornblum, New York and a ruthless dictator-which declared of experimentation the participants London, Routledge, 1998, 297 pages, whole subgroups within human soci- were angry when two of their col- £19.99. ety to have "lives not worth living". leagues testified against the experi- The willing complicity of many Ger- ments before congress (page 198). Acres of Skin presents an angry, mans and German physicians with However imperfect the consent might distressing and provoking description these policies remains a huge warning have been, we must conclude that of human experimentation within the to all of us. Nevertheless, it was in the consent was obtained for these experi- American prison system. Specifically, context of the totalitarian govern- ments. it focuses upon experiments con- ments that the great transgressions Hornblum suggests that many pris- ducted by investigators from the Uni- against human dignity occurred in the oners were injured by their participa- versity of Pennsylvania at the nearby Holmesburg Prison, from 1951 until twentieth century. In Nazi Germany tion, but does not objectively docu- experiments designed to involve severe ment the extent and seriousness of 1974. The research was halted follow- ing congressional hearings in 1973 suffering, often to end in the death of such injuries. -
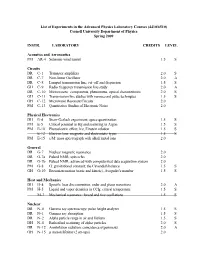
List of Experiments in the Advanced Physics Laboratory Courses (4410/6510) Cornell University Department of Physics Spring 2009
List of Experiments in the Advanced Physics Laboratory Courses (4410/6510) Cornell University Department of Physics Spring 2009 INSTR. LABORATORY CREDITS LEVEL Acoustics and Aeronautics PM AR-4 Subsonic wind tunnel 1.5 S Circuits DR C-1 Transistor amplifiers 2.0 S DR C-7 Non-linear Oscillator 2.0 A DR C-8 Lumped transmission line; cut-off and dispersion 1.5 S GH C-9 Radio frequency transmission line study 2.0 A DR C-10 Microwaves: components, phenomena, optical characteristics 2.0 S GH C-11 Transmission line studies with nanosecond pulse techniques 1.5 S GH C-12 Microwave Resonant Circuits 2.0 PM C-13 Quantitative Studies of Electronic Noise 2.0 Physical Electronics DH E-4 Stern-Gerlach experiment, space quantization 1.5 S PM E-5 Critical potential in Hg and scattering in Argon 1.5 S PM E-10 Photoelectric effect; h/e, Einstein relation 1.5 S E-12 Electron lens: magnetic and electrostatic types 1.5 S PM E-15 e/M: mass spectrograph with alkali metal ions 2.0 General DR G-7 Nuclear magnetic resonance 2.0 DR G-7a Pulsed NMR; spin echo 2.0 DR G-7b Pulsed NMR; advanced with computerized data acquisition system 2.0 PM G-8 G: gravitational constant; the Cavendish balance 1.5 S GH G-10 Brownian motion (static and kinetic), Avogadro's number 1.5 S Heat and Mechanics DH H-4 Specific heat discontinuities; order and phase transitions 2.0 A PM H-5 Liquid and vapor densities in CCl4; critical temperature 1.5 S M-3 Mechanical resonance: forced and free oscillations 1.5 S Nuclear DR N-0 Gamma ray spectroscopy: pulse height analyzer 1.5 S DR N-1 Gamma