Wo 2007/087452 A2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Discriminative Stimulus Effects of Cyclorphan: Selective Antagonism with Naltrexone
Psychopharmacology (1992) 106:189-194 Psychopharmacology Springer-Verlag 1992 Discriminative stimulus effects of cyclorphan: selective antagonism with naltrexone Albert J. Berta|mio and James H. Woods Departments of Psychology and Pharmacology, University of Michigan, Ann Arbor, MI 48109-0626, USA Received June 4, 1990 / Final version September 13, 1990 Abstract. The opioid antagonist, naltrexone, was used to to discriminate ethylketazocine (EKC) yielded high levels identify some of the receptor mechanisms responsible for of EKC-appropriate responding. Nevertheless, the levels the discriminative stimulus effects of cyclorphan in the that were attained with/-cyclorphan were not as high as pigeon. Subjects were trained to discriminate 10 mg/kg those that were readily obtainable with EKC itself, i.e., IM injections of either morphine or dextrorphan from there was a "ceiling" effect. Thus, that study suggested saline injections in a two key drug discrimination that /-cyclorphan shares some properties with opioid procedure in which responding was maintained by food agonists, but it also suggested that/ocyclorphan differs presentation. The dextrorphan-trained birds generalized from drugs in the opioid agonist class in some way. In to/-cyclorphan at 10 mg/kg; naltrexone did not alter the another phase of the previous study pigeons were chron- /-cyclorphan dose-response curve for this effect. In the ically treated with morphine and trained to discriminate morphine-trained group, l-cyclorphan produced only injections of naltrexone. The morphine-treated nal- partial generalization, and naltrexone greatly increased trexone-trained pigeons generalized fully to /-cyclor- the dose of/-cyclorphan necessary to produce this effect. phan, indicating that /-cyclorphan also shares some These results are consistent with the conclusion that in properties with drugs in the opioid antagonist class. -

IS CIRCUMCISION a FRAUD? FRAUD? a IS CIRCUMCISION Peter W
cjp_30-1_42664 Sheet No. 27 Side A 11/12/2020 09:05:36 \\jciprod01\productn\C\CJP\30-1\CJP102.txt unknown Seq: 1 11-NOV-20 14:50 IS CIRCUMCISION A FRAUD? Peter W. Adler, Robert Van Howe, Travis Wisdom & Felix Daase* This Article suggests that non-therapeutic male circumcision or male genital cutting (MGC), the irreversible removal of the foreskin from the penises of healthy boys, is not only unlawful in the United States but also fraudulent. As a German court held in 2012 before its ruling was effectively overturned by a special statute under political pressure, cir- cumcision for religious or non-medical reasons is harmful, violates the child’s rights to bodily integrity and self-determination (which super- sedes competing parental rights), and constitutes criminal assault. MGC also violates the child’s rights under U.S. law, and it constitutes a bat- tery, a tort and a crime, and statutory child abuse. Building upon a 2016 case in the United Kingdom, we make the novel suggestion that when performed by a physician, MGC is a breach of trust or fiduciary duty, and hence constructive fraud, where courts impute fraud even if intent to defraud is absent. We reprise and build upon the argument that it is unlawful and Medicaid fraud for physicians and hospitals to bill Medi- caid for unnecessary genital surgery. Finally, we suggest that MGC con- stitutes intentional fraud by the American Academy of Pediatrics (AAP) and most physicians who perform circumcisions in the United States. They have long portrayed MGC as medicine when it is violence, and as a parental right when males have the right to keep their penile foreskin, and physicians are not allowed to take orders from parents to perform cjp_30-1_42664 Sheet No. -

(12) United States Patent (10) Patent No.: US 9,687,445 B2 Li (45) Date of Patent: Jun
USOO9687445B2 (12) United States Patent (10) Patent No.: US 9,687,445 B2 Li (45) Date of Patent: Jun. 27, 2017 (54) ORAL FILM CONTAINING OPIATE (56) References Cited ENTERC-RELEASE BEADS U.S. PATENT DOCUMENTS (75) Inventor: Michael Hsin Chwen Li, Warren, NJ 7,871,645 B2 1/2011 Hall et al. (US) 2010/0285.130 A1* 11/2010 Sanghvi ........................ 424/484 2011 0033541 A1 2/2011 Myers et al. 2011/0195989 A1* 8, 2011 Rudnic et al. ................ 514,282 (73) Assignee: LTS Lohmann Therapie-Systeme AG, Andernach (DE) FOREIGN PATENT DOCUMENTS CN 101703,777 A 2, 2001 (*) Notice: Subject to any disclaimer, the term of this DE 10 2006 O27 796 A1 12/2007 patent is extended or adjusted under 35 WO WOOO,32255 A1 6, 2000 U.S.C. 154(b) by 338 days. WO WO O1/378O8 A1 5, 2001 WO WO 2007 144080 A2 12/2007 (21) Appl. No.: 13/445,716 (Continued) OTHER PUBLICATIONS (22) Filed: Apr. 12, 2012 Pharmaceutics, edited by Cui Fude, the fifth edition, People's Medical Publishing House, Feb. 29, 2004, pp. 156-157. (65) Prior Publication Data Primary Examiner — Bethany Barham US 2013/0273.162 A1 Oct. 17, 2013 Assistant Examiner — Barbara Frazier (74) Attorney, Agent, or Firm — ProPat, L.L.C. (51) Int. Cl. (57) ABSTRACT A6 IK 9/00 (2006.01) A control release and abuse-resistant opiate drug delivery A6 IK 47/38 (2006.01) oral wafer or edible oral film dosage to treat pain and A6 IK 47/32 (2006.01) substance abuse is provided. -

(12) United States Patent (10) Patent No.: US 8,231,900 B2 Palmer Et Al
USOO823 1900B2 (12) United States Patent (10) Patent No.: US 8,231,900 B2 Palmer et al. (45) Date of Patent: *Jul. 31, 2012 (54) SMALL-VOLUME ORAL TRANSMUCOSAL 4,873,076 A 10, 1989 Fishman et al. DOSAGE 4,880,634 A 1 1/1989 Speiser et al. 5,080,903. A 1/1992 Ayache 5,112,616 A 5/1992 McCartv et al. (75) Inventors: Pamela Palmer, San Francisco, CA 5, 122,127 A 6, 1992 St. (US); Thomas Schreck, Portola Valley, 5,132,114 A 7/1992 Stanley CA (US); Stelios Tzannis, Newark, CA 5,178,878 A 1/1993 Wehling (US); Larry Hamel, Mountain View, CA 5,223,264 A 6, 1993 Wehling et al. O O 5,236,714 A 8, 1993 Lee (US); Andrew I. Poutiatine, San 5,288.497 A 2, 1994 Stanley Anselmo, CA (US) 5,288.498 A 2/1994 Stanley 5,296,234 A 3/1994 Hadaway (73) Assignee: Acelrx Pharmaceutical, Inc., Redwood 5,348,158 A 9, 1994 Honan et al. City, CA (US) 5,489,689 A * 2/1996 Mathew ........................ 546,242 s 5,507,277 A 4, 1996 Rubsamen et al. (*) Notice: Subject to any disclaimer, the term of this 3.68 A SE Exitl patent is extended or adjusted under 35 5,710,551 A 1/1998 Ridgeway et al. U.S.C. 154(b) by 229 days. 5,724,957 A 3, 1998 RubSamen et al. 5,735,263 A 4/1998 RubSamen et al. This patent is Subject to a terminal dis- 5,752,620 A 5/1998 Pearson claimer. -

“I'm Not Missing Anything by Being Circumcised; Why Should I Restore
“I’m not missing anything by being Pleasure. The foreskin is a unique structure filled with which they had always believed was irreversible. circumcised; why should I restore?” delicate nerves and a rich blood supply. The foreskin enhances sexual pleasure, especially as it glides over the Resentment. A 1991 survey of 301 males seeking corona (ridge of the glans) during sexual activity. restoration information showed that almost 70% of those With no accurate means of comparison, the typical circumcised as infants or children resent their parents for circumcised man does not know what he is missing. A Sensitivity. The foreskin is a highly nerve-laden structure, their circumcision. Regaining power over their bodies man, colorblind from birth and thinking his sight is containing approximately 10,000 nerve endings. It is this reduces resentment. normal, might also never question his condition. structure that gives the man his most pleasurable However, as a man ages, he loses sensitivity of the penis. sensations. It also helps to retain glans sensitivity. Empowerment. Victims of rape, crime and child or Many men have difficulty achieving sufficient Circumcision removed this structure and over time spousal abuse typically report a deep sense of stimulation to reach orgasm. The foreskin is a definite sensitivity decreases, making it more difficult to achieve helplessness and vulnerability. Who is more helpless and asset in maintaining this sensitivity. satisfactory stimulation. vulnerable than a restrained newborn having part of his penis amputated? Men restore to take back control of Foreskin restoration is a logical process of returning the Lubrication. Much as the way your eyelid lubricates and their bodies from the damage done by parents, their penis to as close to its original condition as possible. -

Owners Manual
Penisownersinstructionmanual INTRODUCTION Welcome to the world-wide family of penis owners. We trust that you will enjoy many years of trouble-free service from yours. To help ensure that you will, we encourage you to familiarise yourself with the following equipment descriptions, operating instructions and maintenance requirements. BODY AND INTERIOR SPECIFICATIONS (all model years) PENIS Average length and diameter (flaccid) 3.5 x 1.25 inches Average length and diameter (erect) 5.1 x 1.6 inches Average percent increase in volume, flaccid to erect 300% (wow!) Longest medically recorded erection 12 inches Amount of blood in erect penis 8 to 10 x normal Average erections per night (while sleeping) 5 Average duration of each nocturnal erection 20 to 30 minutes Estimated replacement value (good condition model) £50,000 CAUTION! The following can shrink a relaxed penis by two inches or more:- Cold weather, chilly baths or showers, sexual activity, exhaustion, excitement (non sexual), illness and Richard Branson. TESTICLES Average length and width 1.4 x 1 inch Average weight 0.875 x 1.75 ounces Temperature 94.6 degrees Fahrenheit Compartments within each 400 SPERM Average body's production 50,000 per minute/72 million per day (and remember lads, it only takes 1!) Days to maturity 84 Number in ejaculate of average fertile 200 to 600 million man Number of ejaculate of infertile man less than 50 million Percentage of total ejaculate 3% Average swimming speed 1 to 4 millimetres per minute Average life span once mature 1 month in you, 1 to 2 days in woman, 2 minutes on sheets CAUTION! The testicles need to be slightly cooler than normal body temperature for optimum sperm production. -

European Urology Today Official Newsletter of the European Association of Urology Vol
European Urology Today Official newsletter of the European Association of Urology Vol. 25 No.6/Vol. 26 No.1 - Dec. 2013/Jan. 2014 What to expect in Stockholm 9th SEEM in Greece From max to mini Get a preview of what’s on during the From ‘country debates’ to research awards, Needlescopic surgery is back and the interest is 4/5 EAU Congress in Stockholm 14 the SEEM takes a new turn 30 growing Urologists, medical and radiation oncologists come together 5th EMUC in Marseille gathers delegates from 61 countries By Loek Keizer Over the course of three days, three separate but intrinsically linked disciplines attended a scientific meeting and exchanged views on the treatment of radiotherapists approach tumours with probabilities urological cancers. The 5th edition of the European and sigmoid curves. I’m convinced that by learning Multidisciplinary Meeting on Urological Cancers each other’s language and working together in (EMUC) was organised by the EAU, the European multidisciplinary teams is the future for the treatment Society for Medical Oncology (ESMO) and the European of urological cancers.” Society for Radiotherapy and Oncology (ESTRO). On the current state of urological cancer treatment: The meeting took place at in Marseille from 15-17 “We shouldn’t be working as separate, sometimes November, at the capable facilities of the Palais des opposing columns within medicine: collaboration is Congrès at the Parc Chanot. The congress centre was a vital to receive funding and improve care for the brief boulevard stroll away from the Mediterranean patient. We are starting to see multidisciplinary coast, with a temperate breeze being a warm clinics in the United States, where urologists buy their welcome to visitors from Northern Europe. -

Non-Surgical Foreskin Restoration
February 2016 Non-surgical Foreskin Restoration A publication of Doctors Opposing Circumcision www.doctorsopposingcircumcision.org Seattle, Washington Foreskin restoration cannot truly replicate what was stolen, but it can and does provide a vast improvement on the circumcised state. Re-covering my glans improved my sexual function, my body image, and my sense of well-being. There was also a sense of alchemy, of personal victory over something that had seemed hopeless. ‒ J.L., Vancouver, B.C., born 1954 A significant number of circumcised men – in the U.S. and around the world – feel loss, resentment, betrayal, and anger over the violation of their bodies at birth. It takes only a minimal understanding of normal anatomy and function of the normal penis, and of the tawdry social history of circumcision in English language medicine, to understand these feelings of regret and the motivation these men have to restore. Doctors Opposing Circumcision heartily recommends foreskin restoration for those who feel drawn to restore. We estimate that more than 100,000 North American men have completed or are currently engaged in non-surgical foreskin restoration. We are available to advise those of our medical colleagues who encounter patients interested in non-surgical foreskin restoration. We will also gladly counsel men who have questions about restoration, or refer them to a knowledgeable medical professional in their region. Restoration, a safe and proven technique, does not require medical monitoring; however, a restorer may seek medical reassurance. D.O.C. is not alone in recommending against surgical foreskin restoration,[1] which may, we believe, lead to further scar tissue and nerve damage. -

General Agreement on Tariffs Andtrade
RESTRICTED GENERAL AGREEMENT TAR/W/87/Rev.1 16 June 1994 ON TARIFFS AND TRADE Limited Distribution (94-1266) Committee on Tariff Concessions HARMONIZED COMMODITY DESCRIPTION AND CODING SYSTEM (Harmonized System) Classification of INN Substances Revision The following communication has been received from the Nomenclature and Classification Directorate of the Customs Co-operation Council in Brussels. On 25 May 1993, we sent you a list of the INN substances whose classification had been discussed and decided by the Harmonized System Committee. At the time, we informed you that the classification of two substances, clobenoside and meclofenoxate, would be decided later. Furthermore, for some of the chemicals given in that list, one of the contracting parties had entered a reservation and the Harmonized System Committee therefore reconsidered its earlier decision in those cases. I am therefore sending you herewith a revised complete list of the classification decisions of the INN substances. In this revised list, two substances have been added and the classifications of two have been revised as explained below: (a) Addition Classification of clobenoside, (subheading 2940.00) and meclofenoxate (subheading 2922.19). (b) Amendment Etafedrine and moxidentin have now been reclassified in subheadings 2939.40 and 2932.29 respectively. The list of INN substances reproduced hereafter is available only in English. TAR/W/87/Rev. 1 Page 2 Classification of INN Substances Agreed by the Harmonized System Committee in April 1993 Revision Description HS Code -

In Silico Results of Κ-Opioid Receptor Antagonists As Ligands for The
bioRxiv preprint doi: https://doi.org/10.1101/432468; this version posted October 3, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. In silico results of k-Opioid receptor antagonists as ligands for the second bromodomain of the Pleckstrin Homology Domain Interacting Protein Lemmer R. P. EL ASSAL ([email protected]) 25/08/2018 Abstract Pleckstrin Homology Domain Interacting Protein (PHIP) is a member of the BRWD1-3 Family (Bromodomain and WD repeat-containing proteins). PHIP (BRWD2, WDR11) contains a WD40 repeat (methyl-lysine binder) and 2 bromodomains (acetyl-lysine binder). It was discovered through interactions with the pleckstrin homology domain of Insulin Receptor Signalling (IRS) proteins and has been shown to mediate transcriptional responses in pancreatic islet cells and postnatal growth. An initial hit for the second bromodomain of PHIP (PHIP(2)) was discovered in 2012, with consecutive research yielding a candidate with a binding anity of 68mM. PHIP(2) is an atypical category III bromodomain with a threonine (THR1396) where an asparagine residue would usually be. In the standard case, this pocket holds four water molecules, but in the case of PHIP(2), there is room for one extra water molecule - also known as PHIP water, able to mediate interaction between THR1396 and the typical water network at the back of the binding pocket. We present rst ever results of two k-Opioid receptor (KOR) antagonists with distinct pharmacophores having an estimated binding anity in the nM to mM range, as well as higher binding anities for every currently discovered PHIP(2) ligand towards KOR. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0129443 A1 Pettersson (43) Pub
US 20100129443A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0129443 A1 Pettersson (43) Pub. Date: May 27, 2010 (54) NON-ABUSABLE PHARMACEUTICAL Publication Classification COMPOSITION COMPRISING OPODS (51) Int. Cl. A69/20 (2006.01) (76) Inventor: Anders Pettersson, Uppsala (SE) A6IR 9/14 (2006.01) Correspondence Address: 3. ?t C RYAN KROMHOLZ & MANION, S.C. (2006.01) POST OFFICE BOX 266.18 A6IP 25/00 (2006.01) MILWAUKEE, WI 53226 (US) (52) U.S. Cl. ......... 424/465; 424/489: 514/329; 514/282: 424/464 (21) Appl. No.: 12/312,995 (57) ABSTRACT (22) PCT Filed: Dec. 3, 2007 There is provided pharmaceutical compositions for the treat ment of pain comprising a pharmacologically-effective (86). PCT No.: PCT/GB2OOTFOO4627 amount of an opioid analgesic, or a pharmaceutically-accept S371 (c)(1) able salt thereof, presented in particulate form upon the sur (2), (4) Date: Jan. 12, 2010 faces of carrier particles comprising a pharmacologically s e -la?s effective amount of an opioid antagonist, or a O O pharmaceutically-acceptable Salt thereof, which carrier par Related U.S. Application Data ticles are larger in size than the particles of the opioid anal (60) Provisional application No. 60/872,496, filed on Dec. gesic. The compositions are also useful in prevention of 4, 2006. opioid abuse by addicts. US 2010/0129443 A1 May 27, 2010 NON-ABUSABLE PHARMACEUTICAL ing opioid analgesics, which may be administered by a con COMPOSITION COMPRISING OPODS Venient route, for example transmucosally, particularly, as is usually the case, when such active ingredients are incapable of being delivered perorally due to poor and/or variable bio 0001. -
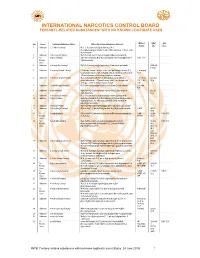
INTERNATIONAL NARCOTICS CONTROL BOARD FENTANYL-RELATED Substancesa with NO KNOWN LEGITIMATE USES
INTERNATIONAL NARCOTICS CONTROL BOARD a FENTANYL-RELATED SUBSTANCES WITH NO KNOWN LEGITIMATE USES Abbrev- CAS Intl No. Uses b Common Substance Name Other/ Alternative Substance Name(s) c iations No.d Ctrl.e 1 Unknown 2,2'-difluorofentanyl N-(1-(2-fluorophenethyl)piperidin-4-yl)-N-(2- fluorophenyl)propionamide; 2'-ortho-difluorofentanyl; 2'-fluoro ortho- fluorofentanyl 2 Unknown 2-fluoro butyrfentanyl N-(2-fluorophenyl)-N-(1-phenethylpiperidin-4-yl) butyramide 3 No 2-fluorofentanyl ortho-fluorofentanyl; N-(2-fluorophenyl)-N-(1-phenethylpiperidin-4- 2-FF; o-FF Known yl)propionamide Uses 4 Unknown 2-furanylethyl fentanyl N-[1-[2-(2-furanyl)ethyl]-4-piperidinyl]-N-phenyl-propanamide 1443-49- 8 (HCl) 5 Unknown 2-isopropylfuranyl fentanyl 2-isopropyl furanyl fentanyl; ortho-isopropyl furanyl fentanyl; N-(2- 2-isopropyl isopropylphenyl)-N-(1-phenethylpiperidin-4-yl)furan-2-carboxamide; Fu-F 2-Furanylfentanyl ortho-2-isopropylphenyl analogue 6 Unknown 2-methoxy furanyl fentanyl N-(2-methoxyphenyl)-N-[1-(2-phenylethyl)-4-piperidinyl]-2- 2-methoxy 101343- furancarboxamide; 2-Furanylfentanyl ortho-2-methoxyphenyl FuF; 2-Meo- 50-4 analogue; ortho-methoxy furanyl fentanyl FuF 7 Unknown 2-methyl furanyl fentanyl N-(1-phenethylpiperidin-4-yl)-N-(o-tolyl)furan-2-carboxamide 2-methyl FuF 8 Unknown 3-allyl fentanyl N-phenyl-N-[1-(2-phenylethyl)-3-(prop-2-en-1-yl)piperidin-4- 82208- yl]propanamide 84-2 9 Unknown 3-fluoro butyrfentanyl N-(3-fluorophenyl)-N-(1-phenethylpiperidin-4-yl) butyramide 10 Unknown 3-fluorofentanyl meta-fluorofentanyl; N-(3-fluorophenyl)-N-(1-phenethylpiperidin-4-