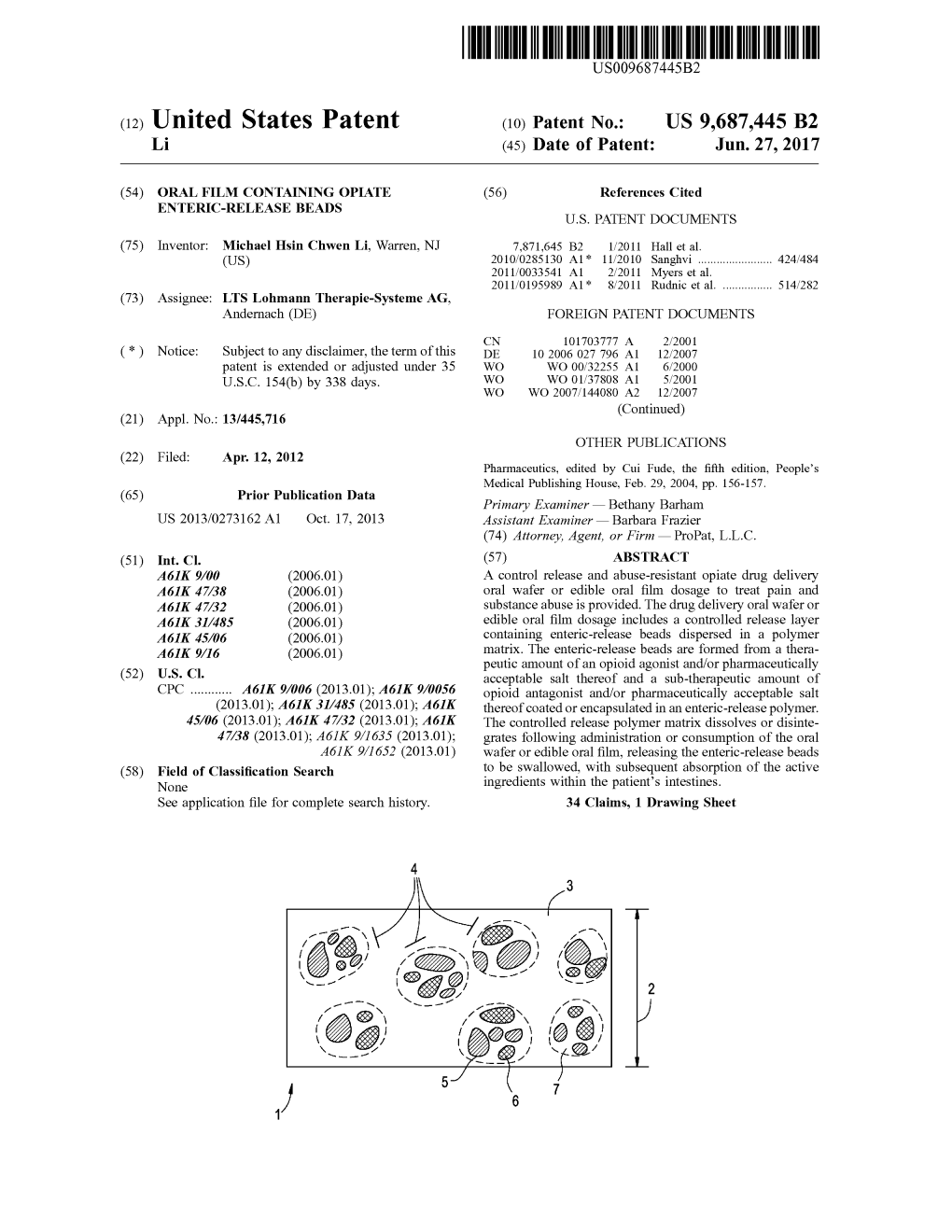
(12) United States Patent (10) Patent No.: US 9,687,445 B2 Li (45) Date of Patent: Jun
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2004/0224020 A1 Schoenhard (43) Pub
US 2004O224020A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0224020 A1 Schoenhard (43) Pub. Date: Nov. 11, 2004 (54) ORAL DOSAGE FORMS WITH (22) Filed: Dec. 18, 2003 THERAPEUTICALLY ACTIVE AGENTS IN CONTROLLED RELEASE CORES AND Related U.S. Application Data IMMEDIATE RELEASE GELATIN CAPSULE COATS (60) Provisional application No. 60/434,839, filed on Dec. 18, 2002. (76) Inventor: Grant L. Schoenhard, San Carlos, CA (US) Publication Classification Correspondence Address: (51) Int. Cl. ................................................... A61K 9/24 Janet M. McNicholas (52) U.S. Cl. .............................................................. 424/471 McAndrews, Held & Malloy, Ltd. 34th Floor (57) ABSTRACT 500 W. Madison Street Chicago, IL 60661 (US) The present invention relates to oral dosage form with active agents in controlled release cores and in immediate release (21) Appl. No.: 10/742,672 gelatin capsule coats. Patent Application Publication Nov. 11, 2004 Sheet 1 of 3 US 2004/0224020 A1 r N 2.S Hr s Patent Application Publication Nov. 11, 2004 Sheet 2 of 3 US 2004/0224020 A1 r CN -8 e N va N . t Cd NOLLYRILNONOO Patent Application Publication Nov. 11, 2004 Sheet 3 of 3 US 2004/0224020 A1 US 2004/0224020 A1 Nov. 11, 2004 ORAL DOSAGE FORMS WITH released formulations, a long t is particularly disadvan THERAPEUTICALLY ACTIVE AGENTS IN tageous to patients Seeking urgent treatment and to maintain CONTROLLED RELEASE CORES AND MEC levels. A second difference in the pharmacokinetic IMMEDIATE RELEASE GELATIN CAPSULE profiles of controlled release in comparison to immediate COATS release drug formulations is that the duration of Sustained plasma levels is longer in the controlled release formula CROSS REFERENCED APPLICATIONS tions. -

Drug Development for the Irritable Bowel Syndrome: Current Challenges and Future Perspectives
REVIEW ARTICLE published: 01 February 2013 doi: 10.3389/fphar.2013.00007 Drug development for the irritable bowel syndrome: current challenges and future perspectives Fabrizio De Ponti* Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy Edited by: Medications are frequently used for the treatment of patients with the irritable bowel syn- Angelo A. Izzo, University of Naples drome (IBS), although their actual benefit is often debated. In fact, the recent progress in Federico II, Italy our understanding of the pathophysiology of IBS, accompanied by a large number of preclin- Reviewed by: Elisabetta Barocelli, University of ical and clinical studies of new drugs, has not been matched by a significant improvement Parma, Italy of the armamentarium of medications available to treat IBS. The aim of this review is to Raffaele Capasso, University of outline the current challenges in drug development for IBS, taking advantage of what we Naples Federico II, Italy have learnt through the Rome process (Rome I, Rome II, and Rome III). The key questions *Correspondence: that will be addressed are: (a) do we still believe in the “magic bullet,” i.e., a very selective Fabrizio De Ponti, Pharmacology Unit, Department of Medical and Surgical drug displaying a single receptor mechanism capable of controlling IBS symptoms? (b) IBS Sciences, University of Bologna, Via is a “functional disorder” where complex neuroimmune and brain-gut interactions occur Irnerio, 48, 40126 Bologna, Italy. and minimal inflammation is often documented: -

Evaluation of in Silico Approach for Prediction of Presence of Opioid Peptides in Wheat
Evaluation of in silico approach for prediction of presence of opioid peptides in wheat This is the Accepted version of the following publication Garg, Swati, Apostolopoulos, Vasso, Nurgali, Kulmira and Mishra, Vijay Kumar (2018) Evaluation of in silico approach for prediction of presence of opioid peptides in wheat. Journal of Functional Foods, 41. 34 - 40. ISSN 1756-4646 The publisher’s official version can be found at https://www.sciencedirect.com/science/article/pii/S1756464617307454 Note that access to this version may require subscription. Downloaded from VU Research Repository https://vuir.vu.edu.au/36577/ 1 1 Evaluation of in silico approach for prediction of presence of opioid peptides in wheat 2 gluten 3 Abstract 4 Opioid like morphine and codeine are used for the management of pain, but are associated 5 with serious side-effects limiting their use. Wheat gluten proteins were assessed for the 6 presence of opioid peptides on the basis of tyrosine and proline within their sequence. Eleven 7 peptides were identified and occurrence of predicted sequences or their structural motifs were 8 analysed using BIOPEP database and ranked using PeptideRanker. Based on higher peptide 9 ranking, three sequences YPG, YYPG and YIPP were selected for determination of opioid 10 activity by cAMP assay against µ and κ opioid receptors. Three peptides inhibited the 11 production of cAMP to varied degree with EC50 values of YPG, YYPG and YIPP were 5.3 12 mM, 1.5 mM and 2.9 mM for µ-opioid receptor, and 1.9 mM, 1.2 mM and 3.2 mM for κ- 13 opioid receptor, respectively. -

INVESTIGATION of NATURAL PRODUCT SCAFFOLDS for the DEVELOPMENT of OPIOID RECEPTOR LIGANDS by Katherine M
INVESTIGATION OF NATURAL PRODUCT SCAFFOLDS FOR THE DEVELOPMENT OF OPIOID RECEPTOR LIGANDS By Katherine M. Prevatt-Smith Submitted to the graduate degree program in Medicinal Chemistry and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. _________________________________ Chairperson: Dr. Thomas E. Prisinzano _________________________________ Dr. Brian S. J. Blagg _________________________________ Dr. Michael F. Rafferty _________________________________ Dr. Paul R. Hanson _________________________________ Dr. Susan M. Lunte Date Defended: July 18, 2012 The Dissertation Committee for Katherine M. Prevatt-Smith certifies that this is the approved version of the following dissertation: INVESTIGATION OF NATURAL PRODUCT SCAFFOLDS FOR THE DEVELOPMENT OF OPIOID RECEPTOR LIGANDS _________________________________ Chairperson: Dr. Thomas E. Prisinzano Date approved: July 18, 2012 ii ABSTRACT Kappa opioid (KOP) receptors have been suggested as an alternative target to the mu opioid (MOP) receptor for the treatment of pain because KOP activation is associated with fewer negative side-effects (respiratory depression, constipation, tolerance, and dependence). The KOP receptor has also been implicated in several abuse-related effects in the central nervous system (CNS). KOP ligands have been investigated as pharmacotherapies for drug abuse; KOP agonists have been shown to modulate dopamine concentrations in the CNS as well as attenuate the self-administration of cocaine in a variety of species, and KOP antagonists have potential in the treatment of relapse. One drawback of current opioid ligand investigation is that many compounds are based on the morphine scaffold and thus have similar properties, both positive and negative, to the parent molecule. Thus there is increasing need to discover new chemical scaffolds with opioid receptor activity. -

In Vivo and in Vitro Characterization of Naltrindole-Derived
Journal of Psychopharmacology http://jop.sagepub.com/ In vivo and in vitro characterization of naltrindole-derived ligands at the κ-opioid receptor Joseph J Casal-Dominguez, Mary Clark, John R Traynor, Stephen M Husbands and Sarah J Bailey J Psychopharmacol 2013 27: 192 originally published online 31 October 2012 DOI: 10.1177/0269881112464828 The online version of this article can be found at: http://jop.sagepub.com/content/27/2/192 Published by: http://www.sagepublications.com On behalf of: British Association for Psychopharmacology Additional services and information for Journal of Psychopharmacology can be found at: Email Alerts: http://jop.sagepub.com/cgi/alerts Subscriptions: http://jop.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav >> Version of Record - Jan 23, 2013 OnlineFirst Version of Record - Oct 31, 2012 What is This? Downloaded from jop.sagepub.com at University of Bath on May 14, 2013 JOP27210.1177/0269881112464828Journal of PsychopharmacologyCasal-Dominguez et al. 4648282013 Original Paper In vivo and in vitro characterization of naltrindole-derived ligands at the κ-opioid receptor Journal of Psychopharmacology 27(2) 192 –202 © The Author(s) 2013 Reprints and permission: sagepub.co.uk/journalsPermissions.nav 1 2 2 DOI: 10.1177/0269881112464828 Joseph J Casal-Dominguez , Mary Clark , John R Traynor , jop.sagepub.com Stephen M Husbands1 and Sarah J Bailey1 Abstract Accumulating evidence supports a role for κ−opioid receptor antagonists in the treatment of mood disorders. Standard κ-antagonists have an unusual pharmacodynamic action, with a single injection blocking receptor signaling for several weeks. Here, we have characterized the κ-selective properties of two ligands, 5’-(2-aminomethyl) naltrindole (5’-AMN) and N-((Naltrindol-5-yl) methyl) pentanimidamide (5’-MABN), to identify whether modifications of the naltrindole side chain produces short-acting κ-antagonists. -

Peripheral Kappa Opioid Receptor Activation Drives Cold Hypersensitivity in Mice
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.04.325118; this version posted October 4, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Peripheral kappa opioid receptor activation drives cold hypersensitivity in mice Manish K. Madasu1,2,3, Loc V. Thang1,2,3, Priyanka Chilukuri1,3, Sree Palanisamy1,2, Joel S. Arackal1,2, Tayler D. Sheahan3,4, Audra M. Foshage3, Richard A. Houghten6, Jay P. McLaughlin5.6, Jordan G. McCall1,2,3, Ream Al-Hasani1,2,3 1Center for Clinical Pharmacology, St. Louis College of Pharmacy and Washington University School of Medicine, St. Louis, MO, USA. 2Department of Pharmaceutical and Administrative Sciences, St. Louis College of Pharmacy, St. Louis, MO, USA 3Department of Anesthesiology, Pain Center, Washington University. St. Louis, MO, USA. 4 Division of Biology and Biomedical Science, Washington University in St. Louis, MO, USA 5Department of Pharmacodynamics, University of Florida, Gainesville, FL, USA 6Torrey Pines Institute for Molecular Studies, Port St. Lucie, FL, USA Corresponding Author: Dr. Ream Al-Hasani Center for Clinical Pharmacology St. Louis College of Pharmacy Washington University School of Medicine 660 South Euclid Campus Box 8054 St. Louis MO, 63110 [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.10.04.325118; this version posted October 4, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Curriculum Vitae: Lin Chang
CURRICULUM VITAE LIN CHANG, M.D. Professor of Medicine Vatche and Tamar Manoukian Division of Digestive Diseases David Geffen School of Medicine at UCLA PERSONAL HISTORY: Office Address: G. Oppenheimer Center for Neurobiology of Stress and Resilience 10833 Le Conte Avenue CHS 42-210 Los Angeles, CA 90095-7378 (310) 206-0192; FAX (310) 825-1919 EDUCATION: 1978 - 1982 University of California, Los Angeles, Degree: B.S. Biochemistry 1982 - 1986 UCLA School of Medicine, Los Angeles, Degree: M.D. 1986 - 1987 Internship: Harbor-UCLA Medical Center, Internal Medicine 1987 - 1989 Residency: Harbor-UCLA Medical Center, Internal Medicine 1989 - 1990 Research Fellowship: Harbor-UCLA Medical Center, Division of Gastroenterology 1990 - 1992 Clinical Fellowship: UCLA Integrated Program, Gastroenterology BOARD CERTIFICATION: ABIM: Internal Medicine 1989 Gastroenterology 1993, recertification 2014 PROFESSIONAL EXPERIENCE: 2006- present Professor of Medicine-in-Residence, Division of Digestive Diseases, David Geffen School of Medicine at UCLA 2000 - 2006 Associate Professor of Medicine, Division of Digestive Diseases, David Geffen School of Medicine at UCLA 1997 - 2000 Assistant Professor of Medicine, Division of Digestive Diseases, UCLA School of Medicine Chang 2 1993 - 1997 Assistant Professor of Medicine-in-Residence, UCLA, Harbor-UCLA Medical Center 1992 - 1993 Associate Consultant, Mayo Clinic, Rochester, Minnesota, Department of Gastroenterology PROFESSIONAL ACTIVITIES: 2017 – present Vice-Chief, Vatche and Tamar Manoukian Division of Digestive -

Design and Synthesis of Cyclic Analogs of the Kappa Opioid Receptor Antagonist Arodyn
Design and synthesis of cyclic analogs of the kappa opioid receptor antagonist arodyn By © 2018 Solomon Aguta Gisemba Submitted to the graduate degree program in Medicinal Chemistry and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. Chair: Dr. Blake Peterson Co-Chair: Dr. Jane Aldrich Dr. Michael Rafferty Dr. Teruna Siahaan Dr. Thomas Tolbert Date Defended: 18 April 2018 The dissertation committee for Solomon Aguta Gisemba certifies that this is the approved version of the following dissertation: Design and synthesis of cyclic analogs of the kappa opioid receptor antagonist arodyn Chair: Dr. Blake Peterson Co-Chair: Dr. Jane Aldrich Date Approved: 10 June 2018 ii Abstract Opioid receptors are important therapeutic targets for mood disorders and pain. Kappa opioid receptor (KOR) antagonists have recently shown potential for treating drug addiction and 1,2,3 4 8 depression. Arodyn (Ac[Phe ,Arg ,D-Ala ]Dyn A(1-11)-NH2), an acetylated dynorphin A (Dyn A) analog, has demonstrated potent and selective KOR antagonism, but can be rapidly metabolized by proteases. Cyclization of arodyn could enhance metabolic stability and potentially stabilize the bioactive conformation to give potent and selective analogs. Accordingly, novel cyclization strategies utilizing ring closing metathesis (RCM) were pursued. However, side reactions involving olefin isomerization of O-allyl groups limited the scope of the RCM reactions, and their use to explore structure-activity relationships of aromatic residues. Here we developed synthetic methodology in a model dipeptide study to facilitate RCM involving Tyr(All) residues. Optimized conditions that included microwave heating and the use of isomerization suppressants were applied to the synthesis of cyclic arodyn analogs. -

Supplementary Materials
Supplementary Materials Hyporesponsivity to mu-opioid receptor agonism in the Wistar-Kyoto rat model of altered nociceptive responding associated with negative affective state Running title: Effects of mu-opioid receptor agonism in Wistar-Kyoto rats Mehnaz I Ferdousi1,3,4, Patricia Calcagno1,2,3,4, Morgane Clarke1,2,3,4, Sonali Aggarwal1,2,3,4, Connie Sanchez5, Karen L Smith5, David J Eyerman5, John P Kelly1,3,4, Michelle Roche2,3,4, David P Finn1,3,4,* 1Pharmacology and Therapeutics, 2Physiology, School of Medicine, 3Centre for Pain Research and 4Galway Neuroscience Centre, National University of Ireland Galway, Galway, Ireland. 5Alkermes Inc., Waltham, Massachusetts, USA. *Corresponding author: Professor David P Finn, Pharmacology and Therapeutics, School of Medicine, Human Biology Building, National University of Ireland Galway, University Road, Galway, H91 W5P7, Ireland. Tel: +353 (0)91 495280 E-mail: [email protected] S.1. Supplementary methods S.1.1. Elevated plus maze test The elevated plus maze (EPM) test assessed the effects of drug treatment on anxiety-related behaviours in WKY and SD rats. The wooden arena, which was elevated 50 cm above the floor, consisted of central platform (10x10 cm) connecting four arms (50x10 cm each) in the shape of a “plus”. Two arms were enclosed by walls (30 cm high, 25 lux) and the other two arms were without any enclosure (60 lux). On the test day, 5 min after the HPT, rats were removed from the home cage, placed in the centre zone of the maze with their heads facing an open arm, and the behaviours were recorded for 5 min with a video camera positioned on top of the arena. -

Kappa Opioid Receptor Regulation of Erk1/2 Map Kinase Signaling Cascade
1 KAPPA OPIOID RECEPTOR REGULATION OF ERK1/2 MAP KINASE SIGNALING CASCADE: MOLECULAR MECHANISMS MODULATING COCAINE REWARD A dissertation presented by Khampaseuth Rasakham to The Department of Psychology In partial fulfillment of the requirements for the degree of Doctor of Philosophy in the field of Psychology Northeastern University Boston, Massachusetts August, 2008 2 KAPPA OPIOID RECEPTOR REGULATION OF ERK1/2 MAP KINASE SIGNALING CASCADE: MOLECULAR MECHANISMS MODULATING COCAINE REWARD by Khampaseuth Rasakham ABSTRACT OF DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Psychology in the Graduate School of Arts and Sciences of Northeastern University, August, 2008 3 ABSTRACT Activation of the Kappa Opioid Receptor (KOR) modulates dopamine (DA) signaling, and Extracellular Regulated Kinase (ERK) Mitogen-Activated Protein (MAP) kinase activity, thereby potentially regulating the rewarding effects of cocaine. The central hypothesis to be tested is that the time-and drug-dependent KOR-mediated regulation of ERK1/2 MAP kinase activity occurs via distinct molecular mechanisms, which in turn may determine the modulation (suppression or potentiation) by KOR effects on cocaine conditioned place preference (CPP). Three studies were performed to test this hypothesis. Study 1 examined the effects of U50,488 and salvinorin A on cocaine reward. In these studies, mice were treated with equianalgesic doses of agonist from 15 to 360 min prior to daily saline or cocaine place conditioning. At time points corresponding with peak biological activity, both agonists produced saline-conditioned place aversion and suppressed cocaine-CPP, effects blocked by the KOR antagonist nor-BNI. However, when mice were place conditioned with cocaine 90 min after agonist pretreatment, U50,488-pretreated mice demonstrated a 2.5-fold potentiation of cocaine-CPP, whereas salvinorin A-pretreated mice demonstrated normal cocaine-CPP responses. -

Opioid Receptorsreceptors
OPIOIDOPIOID RECEPTORSRECEPTORS defined or “classical” types of opioid receptor µ,dk and . Alistair Corbett, Sandy McKnight and Graeme Genes encoding for these receptors have been cloned.5, Henderson 6,7,8 More recently, cDNA encoding an “orphan” receptor Dr Alistair Corbett is Lecturer in the School of was identified which has a high degree of homology to Biological and Biomedical Sciences, Glasgow the “classical” opioid receptors; on structural grounds Caledonian University, Cowcaddens Road, this receptor is an opioid receptor and has been named Glasgow G4 0BA, UK. ORL (opioid receptor-like).9 As would be predicted from 1 Dr Sandy McKnight is Associate Director, Parke- their known abilities to couple through pertussis toxin- Davis Neuroscience Research Centre, sensitive G-proteins, all of the cloned opioid receptors Cambridge University Forvie Site, Robinson possess the same general structure of an extracellular Way, Cambridge CB2 2QB, UK. N-terminal region, seven transmembrane domains and Professor Graeme Henderson is Professor of intracellular C-terminal tail structure. There is Pharmacology and Head of Department, pharmacological evidence for subtypes of each Department of Pharmacology, School of Medical receptor and other types of novel, less well- Sciences, University of Bristol, University Walk, characterised opioid receptors,eliz , , , , have also been Bristol BS8 1TD, UK. postulated. Thes -receptor, however, is no longer regarded as an opioid receptor. Introduction Receptor Subtypes Preparations of the opium poppy papaver somniferum m-Receptor subtypes have been used for many hundreds of years to relieve The MOR-1 gene, encoding for one form of them - pain. In 1803, Sertürner isolated a crystalline sample of receptor, shows approximately 50-70% homology to the main constituent alkaloid, morphine, which was later shown to be almost entirely responsible for the the genes encoding for thedk -(DOR-1), -(KOR-1) and orphan (ORL ) receptors. -

Cannabinoid Transmission in the Prelimbic Cortex Bidirectionally
15642 • The Journal of Neuroscience, September 25, 2013 • 33(39):15642–15651 Behavioral/Cognitive Cannabinoid Transmission in the Prelimbic Cortex Bidirectionally Controls Opiate Reward and Aversion Signaling through Dissociable Kappa Versus -Opiate Receptor Dependent Mechanisms Tasha Ahmad,1 Nicole M. Lauzon,1 Xavier de Jaeger,1 and Steven R. Laviolette1,2,3 Departments of 1Anatomy and Cell Biology, 2Psychiatry, and 3Psychology, The Schulich School of Medicine and Dentistry, University of Western Ontario, London, Ontario, Canada N5Y 5T8 Cannabinoid, dopamine (DA), and opiate receptor pathways play integrative roles in emotional learning, associative memory, and sensory perception. Modulation of cannabinoid CB1 receptor transmission within the medial prefrontal cortex (mPFC) regulates the emotional valence of both rewarding and aversive experiences. Furthermore, CB1 receptor substrates functionally interact with opiate- related motivational processing circuits, particularly in the context of reward-related learning and memory. Considerable evidence demonstrates functional interactions between CB1 and DA signaling pathways during the processing of motivationally salient informa- tion. However, the role of mPFC CB1 receptor transmission in the modulation of behavioral opiate-reward processing is not currently known. Using an unbiased conditioned place preference paradigm with rats, we examined the role of intra-mPFC CB1 transmission during opiate reward learning. We report that activation or inhibition of CB1 transmission within the prelimbic cortical (PLC) division of the mPFC bidirectionally regulates the motivational valence of opiates; whereas CB1 activation switched morphine reward signaling into an aversive stimulus, blockade of CB1 transmission potentiated the rewarding properties of normally sub-reward threshold conditioning doses of morphine. Both of these effects were dependent upon DA transmission as systemic blockade of DAergic transmission prevented CB1-dependent modulation of morphine reward and aversion behaviors.