Updated Status of Bayou Darter, a Narrowly Ranged Endemic in a Geomorphically Active Watershed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2020 Mississippi Bird EA
ENVIRONMENTAL ASSESSMENT Managing Damage and Threats of Damage Caused by Birds in the State of Mississippi Prepared by United States Department of Agriculture Animal and Plant Health Inspection Service Wildlife Services In Cooperation with: The Tennessee Valley Authority January 2020 i EXECUTIVE SUMMARY Wildlife is an important public resource that can provide economic, recreational, emotional, and esthetic benefits to many people. However, wildlife can cause damage to agricultural resources, natural resources, property, and threaten human safety. When people experience damage caused by wildlife or when wildlife threatens to cause damage, people may seek assistance from other entities. The United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services (WS) program is the lead federal agency responsible for managing conflicts between people and wildlife. Therefore, people experiencing damage or threats of damage associated with wildlife could seek assistance from WS. In Mississippi, WS has and continues to receive requests for assistance to reduce and prevent damage associated with several bird species. The National Environmental Policy Act (NEPA) requires federal agencies to incorporate environmental planning into federal agency actions and decision-making processes. Therefore, if WS provided assistance by conducting activities to manage damage caused by bird species, those activities would be a federal action requiring compliance with the NEPA. The NEPA requires federal agencies to have available -

Fish Relationships with Large Wood in Small Streams
Amencan F~sheriesSociety Symposium 37:179-193, 2003 Fish Relationships with Large Wood in Small Streams USDA Forest Service, Southern Research Station, Department ofFisheries and Wildlife Virginia Tech, Blacksburg, Virginia 24060, USA USDA Forest Service, Southern Research Station 1000 Front Street, Oxford, Massachusetts 38655, USA Abstracf.-Many ecological processes are associated with large wood in streams, such as forming habitat critical for fish and a host of other organisms. Wood loading in streams varies with age and species of riparian vegetation, stream size, time since last disturbance, and history of land use. Changes in the landscape resulting from homesteading, agriculture, and logging have altered forest environments, which, in turn, changed the physical and biological characteristics of many streams worldwide. Wood is also important in creating refugia for fish and other aquatic species. Removing wood from streams typically results in loss of pool habitat and overall complexity as well as fewer and smaller individuals of both coldwater and warmwater fish species. The life histories of more than 85 species of fish have some association with large wood for cover, spawning (egg attachment, nest materials), and feeding. Many other aquatic organisms, such as crayfish, certain species of freshwater mus- sels, and turtles, also depend on large wood during at least part of their life cycles. Introduction Because decay rate and probability of displace- ment are a function of size, large pieces have a Large wood can profoundly influence the struc- greater influence on habitat and physical processes ture and function of aquatic habitats from head- than small pieces. In general, rootwads, branches, waters to estuaries. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Stability, Persistence and Habitat Associations of the Pearl Darter Percina Aurora in the Pascagoula River System, Southeastern USA
Vol. 36: 99–109, 2018 ENDANGERED SPECIES RESEARCH Published June 13 https://doi.org/10.3354/esr00897 Endang Species Res OPENPEN ACCESSCCESS Stability, persistence and habitat associations of the pearl darter Percina aurora in the Pascagoula River System, southeastern USA Scott R. Clark1,4,*, William T. Slack2, Brian R. Kreiser1, Jacob F. Schaefer1, Mark A. Dugo3 1Department of Biological Sciences, The University of Southern Mississippi, Hattiesburg, Mississippi 39406, USA 2US Army Engineer Research and Development Center, Environmental Laboratory EEA, Vicksburg, Mississippi 39180, USA 3Mississippi Valley State University, Department of Natural Sciences and Environmental Health, Itta Bena, Mississippi 38941, USA 4Present address: Department of Biology and Museum of Southwestern Biology, University of New Mexico, Albuquerque, New Mexico 87131, USA ABSTRACT: The southeastern United States represents one of the richest collections of aquatic biodiversity worldwide; however, many of these taxa are under an increasing threat of imperil- ment, local extirpation, or extinction. The pearl darter Percina aurora is a small-bodied freshwater fish endemic to the Pearl and Pascagoula river systems of Mississippi and Louisiana (USA). The last collected specimen from the Pearl River drainage was taken in 1973, and it now appears that populations in this system are likely extirpated. This reduced the historical range of this species by approximately 50%, ultimately resulting in federal protection under the US Endangered Species Act in 2017. To better understand the current distribution and general biology of extant popula- tions, we analyzed data collected from a series of surveys conducted in the Pascagoula River drainage from 2000 to 2016. Pearl darters were captured at relatively low abundance (2.4 ± 4.0 ind. -

ECOLOGY of NORTH AMERICAN FRESHWATER FISHES
ECOLOGY of NORTH AMERICAN FRESHWATER FISHES Tables STEPHEN T. ROSS University of California Press Berkeley Los Angeles London © 2013 by The Regents of the University of California ISBN 978-0-520-24945-5 uucp-ross-book-color.indbcp-ross-book-color.indb 1 44/5/13/5/13 88:34:34 AAMM uucp-ross-book-color.indbcp-ross-book-color.indb 2 44/5/13/5/13 88:34:34 AAMM TABLE 1.1 Families Composing 95% of North American Freshwater Fish Species Ranked by the Number of Native Species Number Cumulative Family of species percent Cyprinidae 297 28 Percidae 186 45 Catostomidae 71 51 Poeciliidae 69 58 Ictaluridae 46 62 Goodeidae 45 66 Atherinopsidae 39 70 Salmonidae 38 74 Cyprinodontidae 35 77 Fundulidae 34 80 Centrarchidae 31 83 Cottidae 30 86 Petromyzontidae 21 88 Cichlidae 16 89 Clupeidae 10 90 Eleotridae 10 91 Acipenseridae 8 92 Osmeridae 6 92 Elassomatidae 6 93 Gobiidae 6 93 Amblyopsidae 6 94 Pimelodidae 6 94 Gasterosteidae 5 95 source: Compiled primarily from Mayden (1992), Nelson et al. (2004), and Miller and Norris (2005). uucp-ross-book-color.indbcp-ross-book-color.indb 3 44/5/13/5/13 88:34:34 AAMM TABLE 3.1 Biogeographic Relationships of Species from a Sample of Fishes from the Ouachita River, Arkansas, at the Confl uence with the Little Missouri River (Ross, pers. observ.) Origin/ Pre- Pleistocene Taxa distribution Source Highland Stoneroller, Campostoma spadiceum 2 Mayden 1987a; Blum et al. 2008; Cashner et al. 2010 Blacktail Shiner, Cyprinella venusta 3 Mayden 1987a Steelcolor Shiner, Cyprinella whipplei 1 Mayden 1987a Redfi n Shiner, Lythrurus umbratilis 4 Mayden 1987a Bigeye Shiner, Notropis boops 1 Wiley and Mayden 1985; Mayden 1987a Bullhead Minnow, Pimephales vigilax 4 Mayden 1987a Mountain Madtom, Noturus eleutherus 2a Mayden 1985, 1987a Creole Darter, Etheostoma collettei 2a Mayden 1985 Orangebelly Darter, Etheostoma radiosum 2a Page 1983; Mayden 1985, 1987a Speckled Darter, Etheostoma stigmaeum 3 Page 1983; Simon 1997 Redspot Darter, Etheostoma artesiae 3 Mayden 1985; Piller et al. -

Endangered Species Act Section 7 Consultation Final Programmatic
Endangered Species Act Section 7 Consultation Final Programmatic Biological Opinion and Conference Opinion on the United States Department of the Interior Office of Surface Mining Reclamation and Enforcement’s Surface Mining Control and Reclamation Act Title V Regulatory Program U.S. Fish and Wildlife Service Ecological Services Program Division of Environmental Review Falls Church, Virginia October 16, 2020 Table of Contents 1 Introduction .......................................................................................................................3 2 Consultation History .........................................................................................................4 3 Background .......................................................................................................................5 4 Description of the Action ...................................................................................................7 The Mining Process .............................................................................................................. 8 4.1.1 Exploration ........................................................................................................................ 8 4.1.2 Erosion and Sedimentation Controls .................................................................................. 9 4.1.3 Clearing and Grubbing ....................................................................................................... 9 4.1.4 Excavation of Overburden and Coal ................................................................................ -

Little Bayou Pierre Survey
Little Bayou Pierre Survey Jake Schaefer Department of Biological Sciences University of Southern Mississippi Bayou Darter Research Slack, W. T., Summers, J. A., Rooney, A. P. & Taylor, C. M. 2010 Conservation Genetics of the Threatened Bayou Darter (Percidae: Etheostoma rubrum) in the Bayou Pierre System of Southwestern Mississippi. Copeia 2010, 176–180. Ross, S. T., O’Connell, M., Patrick, D. M., Latorre, C. A., Slack, W. T., Knight, J. G. & Wilkins, S. D. 2001 Stream Erosion and Densities of Etheostoma rubrum (Percidae) and Associated Riffle-Inhabiting Fishes: Biotic Stability in a Variable Habitat. Copeia 2001, 916–927. Suttkus, R. D. & Clemmer, G. H. 1977 A status report on the bayou darter, Etheostoma rubrum, and the Bayou Pierre system. Southeastern Fishes Council Proceedings 1, 1–2. Ross, S. T., Knight, J. G. & Wilkins, S. D. 1992 Distribution and Microhabitat Dynamics of the Threatened Bayou Darter, Etheostoma rubrum. Copeia 1992, 658–671. Knight, J. G. & Ross, S. T. 1992 Reproduction, Age and Growth of the Bayou Darter Etheostoma rubrum (Pisces, Percidae): An Endemic of Bayou Pierre. Am Mid Nat 127, 91–105. Knight, J. G. & Ross, S. T. 1994 Feeding Habits of the Bayou Darter. Trans Am Fish Soc 123, 794–802. Ross, S. T. & Wilkins, S. D. 1993 Reproductive Behavior and Larval Characteristics of the Threatened Bayou Darter (Etheostoma rubrum) in Mississippi. Copeia 1993, 1127–1132. Slack, W. T., Ross, S. T. & Ewing, J. A. 2004 Ecology and population structure of the bayou darter, Etheostoma rubrum: disjunct riffle habitats and downstream -
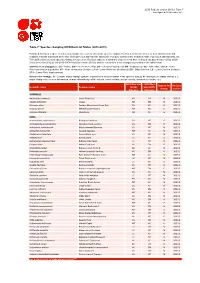
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

The Desperate Dozen: Southeastern Freshwater Fishes on the Brink
Southeastern Fishes Council Proceedings Volume 1 Number 51 Number 51 (December 2009) Article 4 12-1-2009 The Desperate Dozen: Southeastern Freshwater Fishes on the Brink Bernard R. Kuhajda Anna L. George James D. Williams Follow this and additional works at: https://trace.tennessee.edu/sfcproceedings Part of the Marine Biology Commons Recommended Citation Kuhajda, Bernard R.; George, Anna L.; and Williams, James D. (2009) "The Desperate Dozen: Southeastern Freshwater Fishes on the Brink," Southeastern Fishes Council Proceedings: No. 51. Available at: https://trace.tennessee.edu/sfcproceedings/vol1/iss51/4 This Original Research Article is brought to you for free and open access by Volunteer, Open Access, Library Journals (VOL Journals), published in partnership with The University of Tennessee (UT) University Libraries. This article has been accepted for inclusion in Southeastern Fishes Council Proceedings by an authorized editor. For more information, please visit https://trace.tennessee.edu/sfcproceedings. The Desperate Dozen: Southeastern Freshwater Fishes on the Brink This original research article is available in Southeastern Fishes Council Proceedings: https://trace.tennessee.edu/ sfcproceedings/vol1/iss51/4 December 2009 Kuhajda et al. – The Desperate Dozen The Desperate Dozen: Southeastern Freshwater Fishes on the Brink BERNARD R. KUHAJDA 1, ANNA L. GEORGE 2, AND JAMES D. WILLIAMS 3 1Department of Biological Sciences, Box 870345, The University of Alabama, Tuscaloosa, Alabama 35487-0345, Email [email protected]. 2Tennessee Aquarium Conservation Institute, PO Box 11048, Chattanooga, Tennessee 37401-2048, Email [email protected]. 3Florida Museum of Natural History, University of Florida, Museum Road and Newell Drive, Gainesville, Florida 32611, Email [email protected]. -

Age Determination and Growth of Rainbow Darter (Etheostoma Caeruleum) in the Grand River, Ontario
Age Determination and Growth of Rainbow Darter (Etheostoma caeruleum) in the Grand River, Ontario by Alexandra Crichton A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Masters of Science in Biology Waterloo, Ontario, Canada, 2016 ©Alexandra Crichton 2016 Author’s Declaration I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. ii Abstract The accurate determination and validation of age is an important tool in fisheries management. Age profiles allow insight into population dynamics, mortality rates and growth rates, which are important factors in many biomonitoring programs, including the Canadian Environmental Effects Monitoring (EEM) program. Many monitoring studies in the Grand River, Ontario have focused on the impact of municipal wastewater effluent (MWWE) on fish health. Much of the research has been directed at understanding the effects of MWWE on responses across levels of biological organization. The rainbow darter (Etheostoma caeruleum), a small-bodied, benthic fish found throughout the Grand River watershed has been used as a sentinel species in many of these studies. Although changes in somatic indices (e.g. condition, gonad somatic indices) have been included in previous studies, methods to age rainbow darters would provide additional tools to explore impacts at the population level. The objective of the current study was to develop a method to accurately age rainbow darter, validated by use of marginal increment analysis (MIA) and edge analysis (EA) and to characterize growth of male and female rainbow darter at a relatively unimpacted site on the Grand River. -

Fishes of North America Endangered, Threatened, Or of Special Concern: 1979
FISHES OF NORTH AMERICA ENDANGERED, THREATENED, OR OF SPECIAL CONCERN: 1979 James E. Deacon, Gail Kobetich, James D. Williams, Salvador Contreras and Other Members of the Endangered Species Committee of the American Fisheries Society The snail darter, Percina tanasi. Photo by David Etnier of the University of Tennessee. Members of the AFS Endangered Species Committees: Lee Barclay Richard A. Heckmann Alex E. Peden R. Dale Caldwell Clark Hubbs John S. Ramsey Glenn H. Clemmer Gail C. Kobetich Royal D. Suttkus Salvador Contreras James F. La Bounty Charles R. Terrell Walter R. Courtenay, Jr. H. E. McReynolds Phillip L. Wampler James E. Deacon Robert R. Miller Walter R. Whitworth Donald C. Guerre Peter B. Moyle James D. Williams Vincent Guillory Robert J. Naiman James E. Williams Stephen J. Nicola This special section is made possible by the generous financial support of the U.S. Fish and Wildlife Service. March - April 1979 29 FISHES OF NORTH AMERICA ENDANGERED, THREATENED, OR OF SPECIAL CONCERN: 1979 James E. Deacon, Gail Kobetich, James D. Williams, Salvador Contreras and Other Members of the Endangered Species Committee of the American Fisheries Society his list of freshwater fishes—Endangered, Threatened, ing in Monterrey, Nuevo Leon, Mexico, December 6-9, T Of Special Concern—of North America is the result of 1978, he presented his conclusions regarding the status of the three years of effort by the American Fisheries Society (AFS) northern Mexican fishes listed here. The information presented Endangered Species Committee. During 1976 the primary at that Congress will be published as the Memoria del Secundo concern was compiling a list, based on records available Con greso Nacional de Zoologia (Contreras 1978). -

USFWS Consultation on Transmission System Right-Of-Way Program
United States Department of the Interior FISH AND WILDLIFE SERVICE Tennessee ES Office 446 Neal Street Cookeville, Tennessee 38501 December 18, 2018 Mr. John T. Baxter Manager, Biological Compliance Tennessee Valley Authority 400 West Summit Hill Drive Knoxville, TN 37902 Re: FWS #2018-F-0958; Programmatic Consultation for Right-of-Way Vegetation Management that May Affect Endangered or Threatened Plants in the Tennessee Valley Authority Service Area Dear Mr. Baxter: This letter acknowledges the U.S. Fish and Wildlife Service’s (Service) November 21, 2018, receipt of your November 19, 2015, letter requesting initiation of formal section 7 consultation under the Endangered Species Act (Act). The consultation concerns the possible effects of your proposed Programmatic Strategy for Right-of-Way Vegetation Management that May Affect Endangered or Threatened Plants in the Tennessee Valley Authority Service Area (TVA) (the Proposed Action) on 18 federally listed plants, including: • Price's potato-bean (Apios priceana) • Braun's rock-cress (Arabis perstellata) • Pyne's ground plum (Astragalus bibullatus) • Morefield's leather-flower (Clematis morefieldii) • Alabama leather flower (Clematis socialis) • leafy prairie-clover (Dalea foliosa) • whorled sunflower (Helianthus verticillatus) • small whorled pogonia (Isotria medeoloides) • fleshy-fruit gladecress (Leavenworthia crassa) • lyre-leaf bladderpod (Lesquerella lyrata) • Spring Creek bladderpod (Lesquerella perforata) • Mohr's Barbara's buttons (Marshallia mohrii) • Cumberland sandwort (Minuartia cumberlandensis) • Short’s bladderpod (Physaria globosa) • white fringeless orchid (Platanthera integrilabia) • green pitcher plant (Sarracenia oreophila) • large-flowered skullcap (Scutellaria montana) • Tennessee yellow-eyed grass (Xyris tennesseensis) Listed species (LE=listed as endangered; LT=listed as threatened) and designated critical habitats (DCH) that TVA has determined the proposed Action is not likely to adversely affect (NLAA).