Plain English Summary the Families for Health RCT Health Technology Assessment 2017; Vol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Undergraduate Board
1 September 2006 Undergraduate Board To consider QABME: the School of Medicine, University of Leicester for 2005/06 Issue 1. Review of the assessment of Leicester Medical School in the academic year 2005 to 2006. Recommendations 2. The Undergraduate Board are invited to agree: a. In the academic year 2005/06, the School of medicine, University of Leicester met appropriately for that stage the standards of Tomorrow’s Doctors, subject to meeting the requirements in paragraphs 15 a to c. Further Information 3. Coreen Beckford 020 7189 5397 [email protected] Cara Talbot 020 7189 5284 [email protected] Introduction 4. This is the final report to the Education Committee on the quality assurance programme for the School of Medicine, University of Leicester for 2006. 5. The visiting team appointed by the Education Committee to undertake the quality assurance visits included the following individuals. Throughout the rest of this report the GMC visiting team is referred to as the visiting team: Professor Reg Jordan (team leader) Mr Philip Brown Dr Jennie Ciechan Mrs Susan Hobbs Professor Peter McCrorie Dr Philip Milner Professor Trudie Roberts Dr Bruno Rushforth Dr Martin Talbot 6. Miss Coreen Beckford and Ms Cara Talbot supported the visiting team. Our programme of visits in 2005/06 7. The GMC visiting team attended the School on 6 occasions: 14 February 2006, 2 March 2006, 18 May 2006, 23 May 2006, 14 June 2006 and 21 June 2006. 8. The following field work was undertaken: a. Meetings with various members of the School. b. Observation of the clinical examinations. -

Pocketbook for You, in Any Print Style: Including Updated and Filtered Data, However You Want It
Hello Since 1994, Media UK - www.mediauk.com - has contained a full media directory. We now contain media news from over 50 sources, RAJAR and playlist information, the industry's widest selection of radio jobs, and much more - and it's all free. From our directory, we're proud to be able to produce a new edition of the Radio Pocket Book. We've based this on the Radio Authority version that was available when we launched 17 years ago. We hope you find it useful. Enjoy this return of an old favourite: and set mediauk.com on your browser favourites list. James Cridland Managing Director Media UK First published in Great Britain in September 2011 Copyright © 1994-2011 Not At All Bad Ltd. All Rights Reserved. mediauk.com/terms This edition produced October 18, 2011 Set in Book Antiqua Printed on dead trees Published by Not At All Bad Ltd (t/a Media UK) Registered in England, No 6312072 Registered Office (not for correspondence): 96a Curtain Road, London EC2A 3AA 020 7100 1811 [email protected] @mediauk www.mediauk.com Foreword In 1975, when I was 13, I wrote to the IBA to ask for a copy of their latest publication grandly titled Transmitting stations: a Pocket Guide. The year before I had listened with excitement to the launch of our local commercial station, Liverpool's Radio City, and wanted to find out what other stations I might be able to pick up. In those days the Guide covered TV as well as radio, which could only manage to fill two pages – but then there were only 19 “ILR” stations. -

Embedding Physical Activity in the Undergraduate Curriculum
July 2018 Embedding physical activity in the undergraduate curriculum Commissioned by Public Health England (PHE) and Sport England to embed physical activity in the undergraduate curricula in a sample of medical schools and schools of health in England during 2017 and 2018. This is part of the PHE & Sport England’s Moving Healthcare Professionals Programme Authors on behalf of Exercise Works Ltd Ann B. Gates MRPharmS BPharm (Hons) Ian K. Ritchie FRCSEd Executive Summary July 2018 movement for movement Forewords A final year medical student’s view on the lack of exercise medicine in undergraduate curricula As a medical student about to graduate, I have been left disappointed by the lack of exercise medicine I have been exposed to during my time at medical school. Despite it being a well-established way to prevent, treat and manage illness, exercise medicine does not feature significantly in medical curricula throughout the United Kingdom. As a result, there is lack of knowledge and awareness in the medical community. This issue is not limited to doctors: all allied health care professionals are in a position to influence positive lifestyle changes and all should be exposed to exercise medicine in their undergraduate curricula. We are currently missing out on a fantastic opportunity to empower the health care professionals of the future to be confident in advising patients about utilising exercise to improve their health and quality of life. This commission report demonstrates that physical activity can be successfully integrated into medical school curricula, and that students can encourage and support this being implemented. Katie Marino Final year medical student, University of Sheffield. -

The Midlands Is Delivering Nearly £100M of COVID Research to Support the Nation's Fight Against COVID-19 a New Report
The Midlands is delivering nearly £100m of COVID research to support the nation’s fight against COVID-19 A new report - Mobilising Research Excellence in the Midlands to Tackle COVID-19 - published today (Friday, January 15 2021) reveals that the Midlands has moved swiftly to apply its wealth of capability in its hospitals, universities and businesses to deliver £90m of research to support regional, national and global efforts to tackle the Coronavirus Pandemic. The report highlights that: • Experts in the Midlands are leading 81 new COVID-19 research programmes. • The region is playing a crucial and integral role in the world-leading genome sequencing consortium which is identifying the strains of COVID-19 recently in the UK and internationally. • The Midlands has used its internationally leading research excellence and clinical trials infrastructure to recruit over 50,000 patients to COVID-19 clinical trials, driving the discovery of new treatments and scientific insights. • The region has successfully bid for £45m of funding enabling the delivery of £90m of cutting- edge COVID-19 related research. • The region was at the forefront of the early detection of the heightened risks of COVID-19 to the country’s Black and Ethnic Minority population and bringing this to clinical attention. The volume of research projects and clinical trials that the Midlands is not just involved in, but in many cases leading, is exceptional. During the pandemic, the region’s outstanding clinical trials investigators and infrastructure have worked with national organisations to streamline processes and have delivered complex and adaptive clinical trial designs, exceptional recruitment levels and high-quality execution at speeds that were previously thought to be impossible. -

Essential Warwick 2019 Essential Warwick 2019
ESSENTIAL WARWICK 2019 ESSENTIAL WARWICK 2019 27,278 WELCOME Exchange/ TO WARWICK. Visiting, Students Abroad/Industry Warwick is a leading university, and IFP** students somewhere forward-looking and ambitious, where the starting point 1,481 is always ‘anything is possible’. We consistently perform strongly in the UK league tables, and we’re proud to be among the top 20 ‘Most International’ universities in the world*. We’re as respected for boundary-breaking research as for teaching and business collaborations – our pursuit of excellence and intellectual curiosity is tireless. We strive to lead rather than follow, and are renowned for our entrepreneurialism and cosmopolitan outlook. *Times Higher Education, 2018 **International Foundation Programme PLACE Total number of staff OUR (as at 31 March 2019) PEOPLE. 6,947 Total number of students 2018/19 including Academic/Research/ Teaching staff Professional and 27,278 Support staff including 2,610 Undergraduates 4,337 15,998 Postgraduates 9,799 Faculty populations Full-time undergraduate (as % of total student numbers) admissions, October 2018 Applicants Arts % 85% undergraduates12.40 39,974 15% postgraduates Entrants Science Engineering Medicine 5,244 and Medicine 5.73% 54% undergraduates 43.01% 46% postgraduates 63% undergraduates 37% postgraduates Total number of alumni Social Sciences 54% undergraduates 228,080 44.59% 46% postgraduates 3 ESSENTIAL WARWICK 2019 Sport and Wellness Hub OUR CAMPUS.We support a diverse and welcoming community, and we want everyone connected with us to thrive and reach their potential. We’re always looking at ways to improve the campus environment to deliver a space that’s both welcoming and enriching. -

California Dreaming Plans for a University of Warwick Campus in California Have Been Confirmed
fb.com/warwickboar twitter.com/warwickboar theYourboar award-winning student newspaper Wednesday 25th February, 2015 Est. 1973 | Volume 37 | Issue 9 California dreaming Plans for a University of Warwick campus in California have been confirmed » Photo: Warwick University Sonali Gidwani non-profit organisation that claims strategy to develop as a globally to be focused on educating the networked university. American public, has granted War- “A university that exists in many Are you registered? #IVOTE Warwick University have made wick 600 acres of land for the new locations, does research in many plans to open a campus in Cali- campus and funding to take for- locations, and produces global- fornia which will hold 6,000 stu- ward the initiative. ly-aware students able to thrive General Election 2015 dents by 2031. Kyriakos Tsakopoulos, Co-Chair- wherever in the world they decide This follows on from the Busi- man of the University Development to study, research and work. ness School’s campus expansion to Trust, said: “It is thrilling that the “The University of Warwick in the Shard in London in 2014. University of Warwick, one of the California will deliver teaching, Plans for a new overseas campus premier world universities, has tak- and ultimately research, of the were recommended by the Uni- en the first official step to establish highest quality, further extending versity Senate and approved by the a new campus in our region. and accelerating Warwick’s global University Council on Feb 12. “I am confident that Warwick, reach and reputation.” The project will start with de- which achieved astounding world- The University’s Provost, Profes- veloping the University’s teaching class success since its founding in sor Stuart Croft, said: “The Senate presence with a small number of England in 1965, will match that and Council have had a series of postgraduate courses. -

The Guide 2010/2011
the guide 2010/2011 www.warwicksu.com WELCOME TO YOUR UNION On behalf of everyone here at your Students’ Union, I would like to give CONTENTS you the warmest possible welcome to Warwick! 3 Y o u r U n i o n i s t h e h e a r t o f c a m p u s , a n d Welcome aims to not only make sure you have the best experience possible, but to Representation 4 give you opportunities to try things you’ve never even thought of before. There are numerous ways to get Democracy 6 involved, with an endless number of sports clubs, societies, student-run 10 W e l c o m e t o W a r w i c k S t u d e n t s ’ U n i o n a n d y o u r events and officer positions. Your Union definitive guide to what we do, how we do it and Alongside these opportunities, the why you should get involved. The opportunities Union is great for excellent food, drink Welfare 12 for participation are staggering, from clubs and a n d e n t e r t a i n m e n t . C o m e h a v e a b e e r s o c i e t i e s t o d e m o c r a c y a n d v o l u n t e e r i n g , and burger at the Dirty Duck or the best Campaigns 14 campaigning and representation to training hot chocolate on campus at Curiositea. -

Facet Injection Study): a Feasibility Study for a Randomised Controlled Trial
Facet joint injections for people with persistent non-specific low back pain (Facet Injection Study): a feasibility study for a randomised controlled trial David R Ellard,1 Martin Underwood,1* Felix Achana,1 James HL Antrobus,2 Shyam Balasubramanian,3 Sally Brown,4 Melinda Cairns,5 James Griffin,1 Frances Griffiths,6 Kirstie Haywood,7 Charles Hutchinson,8 Ranjit Lall,1 Stavros Petrou,1 Nigel Stallard,9 Colin Tysall,4 David A Walsh10 and Harbinder Sandhu1 1Warwick Clinical Trials Unit, Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK 2South Warwickshire NHS Foundation Trust, Warwick Hospital, Warwick, UK 3Pain Management Service, University Hospital Coventry and Warwickshire, Coventry, UK 4University/User Teaching and Research Action Partnership (UNTRAP), University of Warwick, Coventry, UK 5Department of Allied Health Professions and Midwifery, School of Health and Social Work, University of Hertfordshire, Hatfield, UK 6Social Science and Systems in Health, Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK 7Royal College of Nursing Research Institute, Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK 8Population Evidence and Technologies Room, Warwick Medical School, University of Warwick, University Hospitals of Coventry and Warwickshire, Coventry, UK 9Statistics and Epidemiology, Division of Health Sciences, University of Warwick, Coventry, UK 10Arthritis Research UK Pain Centre, Academic Rheumatology, University of Nottingham, Nottingham, UK *Corresponding author [email protected] Declared competing interests of authors: All authors report grants from the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme during the conduct of the study. Martin Underwood also reports personal fees from the NIHR HTA programme and personal fees from the National Institute for Health and Care Excellence (NICE). -

Mapping the Landscape of UK Health Data Research & Innovation
Mapping the Landscape of UK Health Data Research & Innovation A snapshot of activity in 2017 Dr Ekaterini Blaveri October 2017 CONTENTS Foreword 5 1. Background to this review 7 1.1. Aims 8 1.2. Scope and sections 8 1.3. Approach 9 1.4. Challenges 10 1.5. Acknowledgements 10 2. Overview of major informatics investments by Funder 11 2.1. MRC 12 2.1.1. The Farr Institute of Health Informatics Research 12 2.1.2. MRC Medical Bioinformatics initiative 16 2.1.3. MRC Capital Investment in Genomics England Data Infrastructure 19 2.2.1. The NIHR Biomedical Research Centres 21 2.2.2. The NIHR Health Informatics Collaborative programme 22 2.2.3. The NIHR Collaboration for Leadership in Applied Health Research and Care 25 2.3. Department of Health and Social Care 26 2.3.1. Academic Health Science Centres 26 2.3.2. Genomics England | 100,000 Genomes Project 27 2.4. NHS 28 2.4.1. Academic Health Science Networks 28 2.4.2. NHS Genomic Medicine Centres 30 2.4.3. NHS Global Digital Exemplars 31 2.5. ESRC’s investments in Big Data 32 2.5.1. Administrative Data Research Network 32 2.6. EPSRC’s health data research investments 33 3. Key informatics investments by Research Organisation 35 England – East Anglia and Midlands 38 University of Cambridge 41 EMBL-European Bioinformatics Institute 48 Wellcome Sanger Institute 52 University of Birmingham 56 University of Leicester 62 University of Warwick 68 England – London 74 Imperial College London 76 King’s College London 85 London School of Hygiene and Tropical Medicine 94 Queen Mary University of London 98 University -
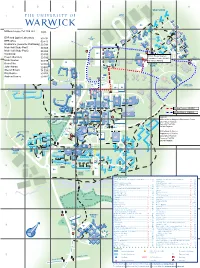
University of Warwick Campus
A B C D E F G H 1 69 46 59 35 30 69 29 53 2 21 68 17 67 71 2 20 70 5 42 18 61 33 40 52 3 32 3 22 62 43 64 24 45 44 1 28 14 37 48 49 4 11 19 66 26 34 55 25 41 65 12 10 47 16 63 15 5 56 60 54 72 H 6 E 57 A L 51 58 T H C E N T R E E R O A D 31 23 27 4 6 50 50 BUILDING KEY International Automotive Research Centre (IARC)..........1 ......E4 Mathematics & Statistics (Zeeman Building) ................37 ...... F4 Arden ...........................................................................2 ...... F2 Maths Houses ............................................................38 ......E8 Argent Court, incorporating Medical Teaching Centre ............................................39 ......D8 Estates, AdsFab & Jobs.ac.uk ......................................3 ..... G3 Millburn House ...........................................................40 ...... F3 Arthur Vick ...................................................................4 ...... F6 Modern Records Centre & BP Archive ........................41 ......D5 Avon Building, incorporating Drama Studio ...................5 ..... G2 Music .........................................................................42 ..... H2 Benefactors ..................................................................6 ..... C5 Nursery .......................................................................43 ..... C3 Biological Sciences .......................................................7 ......D8 Physical Sciences .......................................................44 ......D4 Biomedical Research ....................................................8 -

A Rare Congenital Anemia Is Caused by Mutations in the Centralspindlin Complex 2 3 Sandeep N
medRxiv preprint doi: https://doi.org/10.1101/2021.04.07.21254990; this version posted April 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . 1 A rare congenital anemia is caused by mutations in the centralspindlin complex 2 3 Sandeep N. Wontakal1,5*, Mishan Britto3*, Sarah Tannenbaum2, Benjamin H. 4 Durham1,4, Margaret T. Lee2, Masanori Mishima3,5 5 6 *: co-first authors 7 1: Department of Pathology and Cell Biology 8 2: Department of Pediatrics, Columbia University Irving Medical Center, Columbia 9 University, New York, NY 10032 USA 10 3: Centre for Mechanochemical Cell Biology & Division of Biomedical Sciences, 11 Warwick Medical School, University of Warwick, Coventry CV4 7AL, UK. 12 4: Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY 13 10065 14 5: Corresponding authors: Sandeep N. Wontakal 15 Department of Pathology and Cell Biology 16 Columbia University Irving Medical Center 17 New York, NY 10032 18 Phone: 212-342-4467 19 email: [email protected] 20 21 Masanori Mishima 22 Division of Biomedical Sciences 23 Warwick Medical School 24 University of Warwick 25 Coventry CV4 7AL, UK 26 Phone: +44 (0) 2476 151 928 27 email: [email protected] 28 29 Conflict of interest statement: The authors do not have any conflict of interest to declare. NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

Undergraduate Quality Assurance Visit Report on Warwick Medical
Undergraduate Quality Assurance Visit Report on Warwick Medical School 2011 / 12 Contents Executive summary.................................................................................................. 4 Key findings ............................................................................................................ 6 Risk based visiting.................................................................................................. 8 Concerns raised during the visit ............................................................................. 8 The report ................................................................................................................. 9 Domain 1: Patient safety.......................................................................................... 9 Clinical supervision................................................................................................. 9 Domain 2: Quality assurance, review and evaluation ......................................... 10 Quality management............................................................................................. 10 Agreements with local education providers .......................................................... 10 Evaluation............................................................................................................. 11 Identification and management of risks and concerns.......................................... 12 Domain 3: Equality, diversity and opportunity ...................................................