Evaluation of Processing Variables to Increase the Solids of Milk Protein Concentrates Prior to Drying
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

International Dairy Journal 97 (2019) 111E119
International Dairy Journal 97 (2019) 111e119 Contents lists available at ScienceDirect International Dairy Journal journal homepage: www.elsevier.com/locate/idairyj Physicochemical properties and issues associated with trypsin hydrolyses of bovine casein-dominant protein ingredients * Aaron S.L. Lim, Mark A. Fenelon, Noel A. McCarthy Food Chemistry & Technology Department, Teagasc Food Research Centre, Moorepark, Fermoy, Co. Cork, Ireland article info abstract Article history: Milk protein concentrate (MPC) and sodium caseinate (NaCas) were hydrolysed using the enzyme trypsin Received 25 March 2019 and the subsequent physical properties of the two ingredients were examined. Trypsin hydrolysis was Received in revised form carried out at pH 7 and at 45 C on 11.1% (w/w) protein solutions. Heat inactivation of trypsin was carried 23 May 2019 out when the degree of hydrolysis reached either 10 or 15%. Size-exclusion chromatography and elec- Accepted 23 May 2019 trophoresis confirmed a significant reduction in protein molecular weight in both ingredients. However, Available online 6 June 2019 whey proteins in MPC were more resistant to trypsin hydrolysis than casein. Oil-in-water emulsions were prepared using intact or hydrolysed protein, maltodextrin, and sunflower oil. Protein hydrolysis had a negative effect on the subsequent physical properties of emulsions, compared with non-hydrolysed proteins, with a larger particle size (only for NaCas stabilised emulsions), faster creaming rate, lower heat stability, and increased sedimentation observed in hydrolysed protein emulsions. © 2019 Elsevier Ltd. All rights reserved. 1. Introduction with a protein content of ~90% (w/w) and approximately 1.3% (w/ w) sodium content on a dry basis (Augustin, Oliver, & Hemar, 2011). -

Federal Register/Vol. 82, No. 155/Monday, August 14, 2017
Federal Register / Vol. 82, No. 155 / Monday, August 14, 2017 / Rules and Regulations 37815 times established in advance by a Notice to guidance for industry entitled Management Staff (HFA–305), Food and Airmen. The effective date and time will ‘‘Ultrafiltered Milk in the Production of Drug Administration, 5630 Fishers thereafter be continuously published in the Standardized Cheeses and Related Lane, Rm. 1061, Rockville, MD 20852. Pacific Chart Supplement. Cheese Products: Guidance for • For written/paper comments Paragraph 6004 Class E Airspace Areas Industry.’’ The guidance advises submitted to the Dockets Management Designated as an Extension to a Class D or manufacturers who wish to use Staff, FDA will post your comment, as Class E Surface Area. ultrafiltered milk (UF milk) or well as any attachments, except for * * * * * ultrafiltered nonfat milk (UF nonfat information submitted, marked and AWP HI E4 Hilo, HI [Corrected] milk) in the production of standardized identified, as confidential, if submitted cheeses and related cheese products as detailed in ‘‘Instructions.’’ Hilo International Airport, HI that, pending completion of a (Lat. 19°43′13″ N., long. 155°02′55″ W.) Instructions: All submissions received Hilo VORTAC rulemaking regarding the use of UF milk must include the Docket No. FDA– (Lat. 19°43′17″ N., long. 155°00′39″ W.) in the production of these products, we 2017–D–4713 for ‘‘Ultrafiltered Milk in That airspace extending upward from the intend to exercise enforcement the Production of Standardized Cheeses surface within 3 miles each side of the Hilo discretion regarding the use of fluid UF and Related Cheese Products: Guidance VORTAC 090° radial, extending from the 4.3- milk and fluid UF nonfat milk in the for Industry.’’ Received comments will mile radius of Hilo International Airport to production of standardized cheeses and be placed in the docket and, except for 8.7 miles east of the Hilo VORTAC. -

Trade Focus Dairy Trade and Canada
Trade Focus Last updated A p r i l 2 0 1 7 c onnect to the world of dairy Dairy Trade and Canada The EU Dairy Sector and EDA The European Dairy Association (EDA) is the voice of the milk processing companies, cooperatives and privately owned dairies, at EU level. With 12,000 milk and dairy processing sites across Europe, our sector represents the economic backbone of rural Europe and the industrial basis in many so-called less favoured areas. We @EDA_Dairy | partner daily with over 700,000 dairy farms, accounting for 14% of the whole EU food and drinks industry. Together with over 300,000 industry employees, we all guarantee the high quality of our raw material and our dairy products, which are an essential part of our culinary heritage and of our European cultural treasure. Self-sufficient at 114%, milk and dairy consumption in the EU [email protected] is expected to remain stable while global demand will increase by 1.8% per annum over the | 1 coming decades . While 5 out of the global TOP 10 dairies are headquartered in Europe, the European ‘lactosphère’ is characterised by a tissue of SMEs (small and medium sized enterprises) comprising more than 80% of the total number of dairies in most of the EU Member States. More than €6 bill. were invested over the last years into milk processing capacities in the Union to prepare for the end of the milk quota regime and to be best placed to answer the growing www.euromilk.org/eda global demand. We do support the EU Commission’s efforts to enhance global trade and – as a dairy sector – we are proud to add nearly €10 bill. -

Dairy Stakeholders Praise FDA Ruling on UF Milk Use in Cheese
Volume 37 August 18, 2017 Number 31 Dairy stakeholders praise FDA Like us on Facebook and follow us on Twitter! ruling on UF milk use in cheese A WASHINGTON — Dairy stake- dairy ingredients specifi ed in enforcement discretion until it use this natural, concentrated holders are commending the the standards of identity. has completed its rulemaking form of milk in cheesemaking INSIDE leadership of FDA for granting UF milk is milk that has process or has decided not to with fl exible labeling restric- enforcement discretion for the been fi ltered to remove some proceed with the rulemaking. tions, and the decision will ✦ Half-year cheese use and labeling of ultrafi ltered of the water and lactose, which FDA also notes it is taking open the door for Wisconsin exports up 24 percent. (UF) milk in all standardized increases the protein content this action now due to issues re- and other states to produce For details, see page 3. cheeses and related cheese while reducing total fluid garding domestically-produced and market more fresh, UF products covered by the federal volume. The use of UF milk UF milk in the international milk to cheesemakers across ✦ WCMA launches internship standards of identity. increases effi ciency in cheese- marketplace that have resulted the nation. exchange to enhance FDA in Monday’s Federal making, enhances cheese yield in oversupply and pricing chal- “There’s been an oversupply industry workforce. Register announced the avail- for cheesemakers and allows lenges. of milk in the U.S. for over a year, For details, see page 5. -

High Protein Milk Ingredients - a Tool for Value- Addition to Dairy and Food Products
Journal of Dairy, Veterinary & Animal Research Review Article Open Access High protein milk ingredients - a tool for value- addition to dairy and food products Abstract Volume 6 Issue 1 - 2017 Milk protein ingredients provide not only nutrition, but also specific technological Janki Suthar,1Atanu Jana,2 Smitha functionality when applied in food formulations. Milk protein ingredients are 3 natural, trusted food ingredients and are ideal for unique nutritional and functional Balakrishnan 1Assistant Professor, Department of Dairy Technology, applications. Value addition to dairy and food products are being strived by the dairy Mansinhbhai Institute of Dairy and Food Technology, Mehsana, and food industry to woo more consumers for their innovative products. Use of high India protein milk solids can be one mode of such value-addition to food products. Some 2Department of Dairy Technology, India of the important functional properties of dairy protein ingredients include solubility, 3Assistant Professor, Department of Dairy Chemistry, SMC viscosity building, emulsification, heat stability, aeration, etc. The high protein dairy College of Dairy Science, Anand Agricultural University, India based ingredients highlighted in the review include caseinates, co-precipitates, whey protein concentrates and isolates, milk protein concentrates, micellar casein, UF Correspondence: Atanu Jana, Professor and Head, retentate powder, etc. Cheese analogues are glaring product example in which high Department of Dairy Technology, India, protein ingredients are indispensable. Wheyless cheese can be made utilizing UF Email [email protected] retentate powders. Some of the drawbacks of conventional product such as reduced cheese yield, wheying off from yoghurt, break down of body during storage can be Received: February 28, 2017 | Published: November 14, 2017 taken care of utilizing high protein dairy ingredients in the product formulation. -

Redalyc.Effect of Ph at Drainage on the Physicochemical, Textural and Microstructural Characteristics of Mozzarella Cheese From
Ciência e Tecnologia de Alimentos ISSN: 0101-2061 [email protected] Sociedade Brasileira de Ciência e Tecnologia de Alimentos Brasil PAZ, Noelia Fernanda; GONÇALVEZ DE OLIVEIRA, Enzo; VILLALVA, Fernando Josué; ARMADA, Margarita; RAMÓN, Adriana Noemí Effect of pH at drainage on the physicochemical, textural and microstructural characteristics of mozzarella cheese from goat milk Ciência e Tecnologia de Alimentos, vol. 37, núm. 2, abril-junio, 2017, pp. 193-201 Sociedade Brasileira de Ciência e Tecnologia de Alimentos Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=395951059005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative a Food Science and Technology ISSN 0101-2061 DDOI http://dx.doi.org/10.1590/1678-457X.05116 Effect of pH at drainage on the physicochemical, textural and microstructural characteristics of mozzarella cheese from goat milk Noelia Fernanda PAZ1, Enzo GDNÇALVEZ DE DLIVEIRA1, Fernando Josué VILLALVA1, Margarita ARMADA2, Adriana Noemí RAMÓN3* Abstract The aim of this study was a contribution to standardazation the process of making mozzarella cheese from goat milk by draining at different pH values: 5.0 (MC50), 5.3 (MC53) and 5.6 (MC56), so as to obtain a product with suitable physicochemical, microstructural and textural characteristics. MC50 had lower protein and calcium, with very few strands. MC53 had adequate moisture content, fat, protein and calcium. The cheese yield was higher, the hardness parameters were lower, and the microstructure revealed the presence of long, thin strands, giving it the distinctive texture for this type of cheese. -

Functional and Hydration Properties of Milk Protein Concentrate (MPC)
Milk Science Vol. 64, No. 2 2015 Review Functional and hydration properties of milk protein concentrate (MPC) Shinya Ikeda (Department of Food Science, College of Agricultural and Life Sciences, University of Wisconsin-Madison, 1605 Linden Drive, Madison, WI 53706, USA) Abstract Milk protein concentrates (MPCs) are dried milk protein powders manufactured by membrane ˆltration processes such as ultraˆltration and diaˆltration followed by spray-drying. Due to their excellent nutritional qualities and phys- icochemical functionalities, MPCs are becoming widely recognized for their potential applications in the formulation of a variety of food products including protein-fortiˆed nutritional bars and beverages, and dairy foods such as cheese, yogurt, and ice cream. This review describes functional and hydration properties of MPCs, focusing on its rennet gela- tion behavior, stabilization of oil-in-water emulsions, decreases in the solubility during storage, and potential approaches to remedy the reduced solubility. Similarities and diŠerences in functional properties between MPC and other dairy ingredients such as non fat dry milk (NFDM),skimmilkpowder(SMP), and caseinates are highlighted. The eŠect of temperature and moisture during storage on the development of insolubility of MPC and its implications on the molecular mechanism of the solubility loss are discussed. ultraˆltration of pasteurized skimmed milk6,7). Introduction During this process, caseins and whey proteins are concentrated in the retentate with maintaining Milk protein concentrates (MPCs) are dried their relative proportions, while lactose, milk salts, milk protein powders manufactured by membrane and other small molecules are removed with the ˆltration processes followed by spray-drying1,2). permeate. The ultraˆltration may be followed by MPCs have become widely utilized in a variety of diaˆltration to further increase the mass ratio of food products such as protein bars, nutritional protein to total solids8). -

One Generation Supporting the Next
Annual Report 2017 Report Annual One generation supporting the next. Sustainable Success 1 Here’s what we’ve been doing to help deliver a sustainable future. Contents Chairman’s Report 2-4 CEO’s Report 6-11 Customer Profile – Ausnutria 12-13 Categories and Products 14-15 Redefining Westland’s Purpose 16-17 Shareholder Case Study 18-20 Our Team 22-23 Looking forward to another 80 years 24-27 Environmental Highlights 30-31 Health and Safety 32-34 Five Year Trends 36-37 Board of Directors & Senior Management 38-39 Corporate Governance Report 40-41 Audit Report 42-43 Directors’ Declaration 45 Financial Statements 46-49 Notes to Financial Statements 50-76 Statutory Information 78-79 Directory 80 1 Chairman Pete Morrison We have worked very hard to build The 2016-17 financial year for At the time of writing, the shareholder- Westland Milk Products was initiated governance review process a great future for characterised by challenge and was still underway. It was scheduled change, with new leadership at for presentation to a special meeting Westland. Board and management levels. of shareholders in October 2017. With Westland finishing the 2015-16 year in the unenviable position of Shareholders at the 2016 Annual offering its shareholders the lowest General Meeting, and district meetings payout of any New Zealand dairy in March 2017, asked the Board and company, we began the new financial management team to improve year under considerable financial communications with them. It’s pressure. Understandably, our good to report that recent feedback shareholders were demanding answers suggests this has much improved and and calling for both the Board and shareholders are appreciating receiving management to do much better. -

Role of Milk Protein–Based Products in Some Quality Attributes of Goat Milk Yogurt
J. Dairy Sci. 99 :2694–2703 http://dx.doi.org/ 10.3168/jds.2015-10393 © American Dairy Science Association®, 2016 . Role of milk protein–based products in some quality attributes of goat milk yogurt A. Gursel,*1 A. Gursoy,* E. A. K. Anli,* S. O. Budak,* S. Aydemir,† and F. Durlu-Ozkaya‡ *Department of Dairy Technology, Faculty of Agriculture, Ankara University, 06110 Ankara, Turkey †Enka Dairy and Food Products Industry and Commerce Ltd., 42150 Konya, Turkey ‡Department of Gastronomy and Culinary Arts, Faculty of Tourism, Gazi University, 06830 Gölbaşı, Ankara, Turkey ABSTRACT because of its nutritious and health-beneficial character- istics. Cow, sheep, and goat milk can be used for yogurt Goat milk yogurts were manufactured with the manufacture alone or as a mixture. Sheep milk have fortification of 2% (wt/vol) skim goat milk powder higher TS content than cow and goat milk; therefore, (SGMP), sodium caseinate (NaCn), whey protein con- using sheep milk gives good textural characteristics to centrate (WPC), whey protein isolate (WPI), or yogurt yogurt, whereas yogurt from goat milk presents poor texture improver (YTI). Yogurts were characterized textural characteristics and has a gel structure suscep- based on compositional, microbiological, and textural tible to rupture due mainly to differences in protein properties; volatile flavor components (with gas chro- composition between goat and cow milk (Remeuf and matography); and sensory analyses during storage (21 Lenoir, 1986; Agnihotri and Prasad, 1993; Park et al., d at 5°C). Compared with goat milk yogurt made by 2007; Domagala, 2009). Despite its negative aspects, using SGMP, the other goat milk yogurt variants had goat milk has been regarded as an indispensable raw higher protein content and lower acidity values. -

Modification of Milk Protein Concentrate and Applicability in High-Protein Nutrition Bars Justin Charles Banach Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2012 Modification of milk protein concentrate and applicability in high-protein nutrition bars Justin Charles Banach Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Food Science Commons Recommended Citation Banach, Justin Charles, "Modification of milk protein concentrate and applicability in high-protein nutrition bars" (2012). Graduate Theses and Dissertations. 12786. https://lib.dr.iastate.edu/etd/12786 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. i Modification of milk protein concentrate and applicability in high-protein nutrition bars By Justin Charles Banach A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Food Science and Technology Program of Study Committee: Buddhi P. Lamsal, Major Professor Stephanie Clark James Hollis Iowa State University Ames, Iowa 2012 Copyright © Justin Charles Banach, 2012. All rights reserved. ii TABLE OF CONTENTS LIST OF FIGURES iv LIST OF TABLES v CHAPTER 1. GENERAL INTRODUCTION 1 1.1 Research Premise 1 1.2 Thesis Organization 2 1.3 References 3 CHAPTER 2. -

Progress Report for the Dairy Research Advisory Board
Utah State University DigitalCommons@USU Other Documents Western Dairy Center 7-29-1984 Progress Report for the Dairy Research Advisory Board Various Authors Follow this and additional works at: https://digitalcommons.usu.edu/wdc_otherreports Part of the Dairy Science Commons Recommended Citation Authors, Various, "Progress Report for the Dairy Research Advisory Board" (1984). Other Documents. Paper 3. https://digitalcommons.usu.edu/wdc_otherreports/3 This Report is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Other Documents by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Dairy Research Advisory Board Eccles Conference Center 201-203 Utah State University July 25, 1984 AGENDA 8:30 AM Welcome. Dr. D.J. Matthews, Director, Utah State University Agricultural Experiment Station 8:40 AM PROJECT REVIEWS 1. Calcium and the meltability of process cheese food made from ultrafiltered milk. C.A. Ernstrom 2. Evaluation and improvement of coagulation properties of milk for manufacture. Leslie M. Okigbo 3. Effect of whey protein denaturation in dried whey used for bread making. Ronald Malouf 4. Production of a high moisture white cheese from ultrafiltered milk. S.M.K. Anis 5. Role of milk clotting enzymes in cheese ·curing. C.A. Ernstrom 6. Cottage cheese from ultrafiltered skim milk. Ronald M. Raynes Lunch. Center Colony Room - Taggart Student Center. 7. Plasmid profiles of proteinase positive and negative strains of S. cremoris. Craig J. Oberg 8. Casein/fat ratio and fat recovery in Cheddar cheese. Nana Yiadom-Farkye 9. -
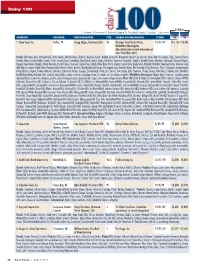
Dairy100 Table.Indd
Dairy 100 In Millions C=Cooperative; JV=Joint venture; Pr=Privately-held company; Pu=Publicly-traded company; S=Subsidiary; *=Sales estimate SALES SALES COMPANY LOCATION DAIRY EXECUTIVE TYPE PARENT CO./SUBSIDIARIES FY END ‘09 ‘08 1. Dean Foods Co. Dallas, TX Gregg Engles, Chairman/CEO Pu Divisions: Fresh Dairy Direct, 12/31/09 $11, 158 $12,454 WhiteWave-Morningstar, Alpro (total sales include international sales from Alpro unit). Brands: Alta Dena, Apro (international), Arctic Splash, Atlanta Dairies, Barber’s, Berkeley Farms, Borden (licensed), Broughton, Brown Cow, Brown’s Dairy, Bud’s Ice Cream, Chug, Country Charm, Country Churn, Country Delite, Country Fresh, Country Love, Creamland, Dairy Fresh, Dean’s, Dipzz, Fieldcrest, Foremost (licensed), Gandy’s, Garelick Farms, Hershey’s (licensed), Horizon Organic, Hygeia, International Delight, Jilbert, Knudsen, Land O’Lakes (licensed), Land-O-Sun, Lehigh Valley Dairy Farms, Liberty, Louis Trauth, Maplehurst, Mayfield, McArthur, Meadow Brook, Meadow Gold, Mile High Ice Cream, Model Dairy, Mountain High, Nature’s Pride, Nurture, Nuttybuddy, Oak Farms, The Organic Cow, Over the Moon, Pet (licensed), Pog (licensed), Price’s, Provamel (international), Purity, Rachel’s Organic, Reiter, Robinson, Saunders, Schenkel’s All Star, Schepps, Shenandoah’s Pride, Silk, Stroh’s, Swiss Dairy, Swiss Premium, TG Lee, Tuscan, Turtle Tracks, Verifine, Viva. Products: Fresh Direct Dairy-Branded milk, cultured, juice/drinks, water, creamers, whipping cream, ice cream mix, ice cream, novelties; WhiteWave-Morningstar-Organic