Federal Register/Vol. 82, No. 155/Monday, August 14, 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dairy Stakeholders Praise FDA Ruling on UF Milk Use in Cheese
Volume 37 August 18, 2017 Number 31 Dairy stakeholders praise FDA Like us on Facebook and follow us on Twitter! ruling on UF milk use in cheese A WASHINGTON — Dairy stake- dairy ingredients specifi ed in enforcement discretion until it use this natural, concentrated holders are commending the the standards of identity. has completed its rulemaking form of milk in cheesemaking INSIDE leadership of FDA for granting UF milk is milk that has process or has decided not to with fl exible labeling restric- enforcement discretion for the been fi ltered to remove some proceed with the rulemaking. tions, and the decision will ✦ Half-year cheese use and labeling of ultrafi ltered of the water and lactose, which FDA also notes it is taking open the door for Wisconsin exports up 24 percent. (UF) milk in all standardized increases the protein content this action now due to issues re- and other states to produce For details, see page 3. cheeses and related cheese while reducing total fluid garding domestically-produced and market more fresh, UF products covered by the federal volume. The use of UF milk UF milk in the international milk to cheesemakers across ✦ WCMA launches internship standards of identity. increases effi ciency in cheese- marketplace that have resulted the nation. exchange to enhance FDA in Monday’s Federal making, enhances cheese yield in oversupply and pricing chal- “There’s been an oversupply industry workforce. Register announced the avail- for cheesemakers and allows lenges. of milk in the U.S. for over a year, For details, see page 5. -

Redalyc.Effect of Ph at Drainage on the Physicochemical, Textural and Microstructural Characteristics of Mozzarella Cheese From
Ciência e Tecnologia de Alimentos ISSN: 0101-2061 [email protected] Sociedade Brasileira de Ciência e Tecnologia de Alimentos Brasil PAZ, Noelia Fernanda; GONÇALVEZ DE OLIVEIRA, Enzo; VILLALVA, Fernando Josué; ARMADA, Margarita; RAMÓN, Adriana Noemí Effect of pH at drainage on the physicochemical, textural and microstructural characteristics of mozzarella cheese from goat milk Ciência e Tecnologia de Alimentos, vol. 37, núm. 2, abril-junio, 2017, pp. 193-201 Sociedade Brasileira de Ciência e Tecnologia de Alimentos Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=395951059005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative a Food Science and Technology ISSN 0101-2061 DDOI http://dx.doi.org/10.1590/1678-457X.05116 Effect of pH at drainage on the physicochemical, textural and microstructural characteristics of mozzarella cheese from goat milk Noelia Fernanda PAZ1, Enzo GDNÇALVEZ DE DLIVEIRA1, Fernando Josué VILLALVA1, Margarita ARMADA2, Adriana Noemí RAMÓN3* Abstract The aim of this study was a contribution to standardazation the process of making mozzarella cheese from goat milk by draining at different pH values: 5.0 (MC50), 5.3 (MC53) and 5.6 (MC56), so as to obtain a product with suitable physicochemical, microstructural and textural characteristics. MC50 had lower protein and calcium, with very few strands. MC53 had adequate moisture content, fat, protein and calcium. The cheese yield was higher, the hardness parameters were lower, and the microstructure revealed the presence of long, thin strands, giving it the distinctive texture for this type of cheese. -

Role of Milk Protein–Based Products in Some Quality Attributes of Goat Milk Yogurt
J. Dairy Sci. 99 :2694–2703 http://dx.doi.org/ 10.3168/jds.2015-10393 © American Dairy Science Association®, 2016 . Role of milk protein–based products in some quality attributes of goat milk yogurt A. Gursel,*1 A. Gursoy,* E. A. K. Anli,* S. O. Budak,* S. Aydemir,† and F. Durlu-Ozkaya‡ *Department of Dairy Technology, Faculty of Agriculture, Ankara University, 06110 Ankara, Turkey †Enka Dairy and Food Products Industry and Commerce Ltd., 42150 Konya, Turkey ‡Department of Gastronomy and Culinary Arts, Faculty of Tourism, Gazi University, 06830 Gölbaşı, Ankara, Turkey ABSTRACT because of its nutritious and health-beneficial character- istics. Cow, sheep, and goat milk can be used for yogurt Goat milk yogurts were manufactured with the manufacture alone or as a mixture. Sheep milk have fortification of 2% (wt/vol) skim goat milk powder higher TS content than cow and goat milk; therefore, (SGMP), sodium caseinate (NaCn), whey protein con- using sheep milk gives good textural characteristics to centrate (WPC), whey protein isolate (WPI), or yogurt yogurt, whereas yogurt from goat milk presents poor texture improver (YTI). Yogurts were characterized textural characteristics and has a gel structure suscep- based on compositional, microbiological, and textural tible to rupture due mainly to differences in protein properties; volatile flavor components (with gas chro- composition between goat and cow milk (Remeuf and matography); and sensory analyses during storage (21 Lenoir, 1986; Agnihotri and Prasad, 1993; Park et al., d at 5°C). Compared with goat milk yogurt made by 2007; Domagala, 2009). Despite its negative aspects, using SGMP, the other goat milk yogurt variants had goat milk has been regarded as an indispensable raw higher protein content and lower acidity values. -

Progress Report for the Dairy Research Advisory Board
Utah State University DigitalCommons@USU Other Documents Western Dairy Center 7-29-1984 Progress Report for the Dairy Research Advisory Board Various Authors Follow this and additional works at: https://digitalcommons.usu.edu/wdc_otherreports Part of the Dairy Science Commons Recommended Citation Authors, Various, "Progress Report for the Dairy Research Advisory Board" (1984). Other Documents. Paper 3. https://digitalcommons.usu.edu/wdc_otherreports/3 This Report is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Other Documents by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Dairy Research Advisory Board Eccles Conference Center 201-203 Utah State University July 25, 1984 AGENDA 8:30 AM Welcome. Dr. D.J. Matthews, Director, Utah State University Agricultural Experiment Station 8:40 AM PROJECT REVIEWS 1. Calcium and the meltability of process cheese food made from ultrafiltered milk. C.A. Ernstrom 2. Evaluation and improvement of coagulation properties of milk for manufacture. Leslie M. Okigbo 3. Effect of whey protein denaturation in dried whey used for bread making. Ronald Malouf 4. Production of a high moisture white cheese from ultrafiltered milk. S.M.K. Anis 5. Role of milk clotting enzymes in cheese ·curing. C.A. Ernstrom 6. Cottage cheese from ultrafiltered skim milk. Ronald M. Raynes Lunch. Center Colony Room - Taggart Student Center. 7. Plasmid profiles of proteinase positive and negative strains of S. cremoris. Craig J. Oberg 8. Casein/fat ratio and fat recovery in Cheddar cheese. Nana Yiadom-Farkye 9. -
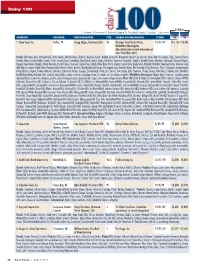
Dairy100 Table.Indd
Dairy 100 In Millions C=Cooperative; JV=Joint venture; Pr=Privately-held company; Pu=Publicly-traded company; S=Subsidiary; *=Sales estimate SALES SALES COMPANY LOCATION DAIRY EXECUTIVE TYPE PARENT CO./SUBSIDIARIES FY END ‘09 ‘08 1. Dean Foods Co. Dallas, TX Gregg Engles, Chairman/CEO Pu Divisions: Fresh Dairy Direct, 12/31/09 $11, 158 $12,454 WhiteWave-Morningstar, Alpro (total sales include international sales from Alpro unit). Brands: Alta Dena, Apro (international), Arctic Splash, Atlanta Dairies, Barber’s, Berkeley Farms, Borden (licensed), Broughton, Brown Cow, Brown’s Dairy, Bud’s Ice Cream, Chug, Country Charm, Country Churn, Country Delite, Country Fresh, Country Love, Creamland, Dairy Fresh, Dean’s, Dipzz, Fieldcrest, Foremost (licensed), Gandy’s, Garelick Farms, Hershey’s (licensed), Horizon Organic, Hygeia, International Delight, Jilbert, Knudsen, Land O’Lakes (licensed), Land-O-Sun, Lehigh Valley Dairy Farms, Liberty, Louis Trauth, Maplehurst, Mayfield, McArthur, Meadow Brook, Meadow Gold, Mile High Ice Cream, Model Dairy, Mountain High, Nature’s Pride, Nurture, Nuttybuddy, Oak Farms, The Organic Cow, Over the Moon, Pet (licensed), Pog (licensed), Price’s, Provamel (international), Purity, Rachel’s Organic, Reiter, Robinson, Saunders, Schenkel’s All Star, Schepps, Shenandoah’s Pride, Silk, Stroh’s, Swiss Dairy, Swiss Premium, TG Lee, Tuscan, Turtle Tracks, Verifine, Viva. Products: Fresh Direct Dairy-Branded milk, cultured, juice/drinks, water, creamers, whipping cream, ice cream mix, ice cream, novelties; WhiteWave-Morningstar-Organic -

Role of Microorganisms Present in Dairy Fermented Products in Health and Disease Neural Computation for Rehabilitation Guest Editors: Clara G
BioMed Research International Role of Microorganisms Present in Dairy Fermented Products in Health and Disease Neural Computation for Rehabilitation Guest Editors: Clara G. de los Reyes-Gavilán, María Fernández, John Andrew Hudson, and Riitta Korpela Role of Microorganisms Present in Dairy Fermented Products in Health and Disease BioMed Research International Role of Microorganisms Present in Dairy Fermented Products in Health and Disease Guest Editors: Clara G. de los Reyes-Gavilan,´ Marc´ıa Fern´ındez, John Andrew Hudson, and Riitta Korpela Copyright © 2015 Hindawi Publishing Corporation. All rights reserved. This is a special issue published in “BioMed Research International.” All articles are open access articles distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Contents Role of Microorganisms Present in Dairy Fermented Products in Health and Disease, Clara G. de los Reyes-Gavilan,´ Marc´ıa Fern´ındez, John Andrew Hudson, and Riitta Korpela Volume 2015, Article ID 204173, 2 pages Antimicrobial Activity of Lactic Acid Bacteria in Dairy Products and Gut: Effect on Pathogens, Juan L. Arques,´ Eva Rodr´ıguez, Susana Langa, JoseMar´ ´ıa Landete, and Margarita Medina Volume2015,ArticleID584183,9pages Impact on Human Health of Microorganisms Present in Fermented Dairy Products: An Overview, Mar´ıa Fernandez,´ John Andrew Hudson, Riitta Korpela, and Clara G. de los Reyes-Gavilan´ Volume 2015, Article ID 412714, 13 pages Bioaccessible Antioxidants in Milk Fermented by Bifidobacterium longum subsp. longum Strains, Merilie´ Gagnon, Patricia Savard, Audrey Riviere,` Gisele` LaPointe, and Denis Roy Volume 2015, Article ID 169381, 12 pages Biodiversity and gamma-Aminobutyric Acid Production by Lactic Acid Bacteria Isolated from Traditional Alpine Raw Cow’s Milk Cheeses, Elena Franciosi, Ilaria Carafa, Tiziana Nardin, Silvia Schiavon, Elisa Poznanski, Agostino Cavazza, Roberto Larcher, and Kieran M. -

Evaluation of Processing Variables to Increase the Solids of Milk Protein Concentrates Prior to Drying
South Dakota State University Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange Electronic Theses and Dissertations 2020 Evaluation of Processing Variables to Increase the Solids of Milk Protein Concentrates Prior to Drying Achyut Mishra South Dakota State University Follow this and additional works at: https://openprairie.sdstate.edu/etd Part of the Dairy Science Commons, and the Food Science Commons Recommended Citation Mishra, Achyut, "Evaluation of Processing Variables to Increase the Solids of Milk Protein Concentrates Prior to Drying" (2020). Electronic Theses and Dissertations. 4989. https://openprairie.sdstate.edu/etd/4989 This Dissertation - Open Access is brought to you for free and open access by Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Open PRAIRIE: Open Public Research Access Institutional Repository and Information Exchange. For more information, please contact [email protected]. EVALUATION OF PROCESSING VARIABLES TO INCREASE THE SOLIDS OF MILK PROTEIN CONCENTRATES PRIOR TO DRYING BY ACHYUT MISHRA A dissertation submitted in partial fulfillment of the requirements for the Doctor of Philosophy Major in Biological Sciences Specialization in Dairy Science South Dakota State University 2020 ii DISSERTATION ACCEPTANCE PAGE ACHYUT MISHRA This dissertation is approved as a creditable and independent investigation by a candidate for the Doctor of Philosophy degree and is acceptable for meeting the dissertation requirements for this degree. Acceptance of this does not imply that the conclusions reached by the candidate are necessarily the conclusions of the major department. Lloyd Metzger Advisor Date Joseph P Cassady Department Head Date Nicole Lounsbery, PhD Director, Graduate School Date iii I am dedicating this dissertation to my beloved mom, Late Januka Devi Mishra. -

2Nd Biennial Ultra-High Temperature (UHT) Symposium
Utah State University DigitalCommons@USU Other Documents Western Dairy Center 3-20-1996 2nd Biennial Ultra-High Temperature (UHT) Symposium Various Authors Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/wdc_otherreports Part of the Food Science Commons Recommended Citation Authors, Various, "2nd Biennial Ultra-High Temperature (UHT) Symposium" (1996). Other Documents. Paper 5. https://digitalcommons.usu.edu/wdc_otherreports/5 This Conference Paper is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Other Documents by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. SECOND BIENNIAL UHT.'S~?OSUJM~ ' Wei~~ln,·Center . ' ... ' ·~.-' ~ Utah State Uni\rersity March 19.!20,.1996 Agenda Tuesday, March 1~ ... ,::-,------:c-:------------ --- 7:30 Registration/ continental breakfast Effect of UHT processing on product 'ingredients - Ch~ir: Za4QW · · 8:30 Don McMahon, USU- Heat effects on ~-lactoglobulin., 9:15 Paul SaveHo, USU- Effect of UHT processing on.flavors 10:00 Break · 10:30 Steven Dimler, Ross Labs- Vitamin and nutrient degradation 11:15 Woodrow Monte, Arizona State University-: Ingredients for UHT- products 12:00 Lunch · · New product developme~t -Chair: Fehling 1:30 Paul Savello, USU- Modifying skim milk using UHT equipm~nc ' ..... '. 2:15 Paul Scharfman, Specialty Cheese Co.- New product marketing· ' .~-~· 3:00 Break 3:30 Pat Fehling, Fehling Associates- Product development • 4:15 Charles Watkins, Robert Bosch Company- UHT packaging options 5:00 Adjourn 6:30 Dinner Wednesday, March 20 Safety and sanitation in UHT systems - Chair: Savello 8:00 Continental breakfast 8:30 Bart Weimer, USU- Milk quality for UHT processing 9:15 Chuck Sizer, Tetra Pak.- Time/temperature relationships and destruction of spores. -

Abstract Schiano, Angelina Nicole
ABSTRACT SCHIANO, ANGELINA NICOLE. Consumer Perception of Dairy Foods: Sustainability and Processing Methods (Under the direction of Dr. MaryAnne Drake). Changing consumer desires have led the growth of new dairy products and plant-based dairy alternatives to better meet these needs. The objective of this dissertation was to better understand changing consumer needs for and perception of dairy products. Four different studies were conducted. In the first study, consumer perception of the sustainability of milk and dried dairy ingredients and their respective plant-based alternatives were characterized using focus groups and two online surveys with dairy product consumers. Focus groups and survey maximum difference scaling results identified 5 key attributes for sustainability: minimal carbon footprint/greenhouse gas emissions, few/no preservatives, animal happiness and welfare, and simple/minimal ingredients. Plant-based dairy alternatives were considered more sustainable than dairy products, but package type and organic status also played a role in consumer sustainability perception. There was cognitive overlap among the terms sustainable, natural, healthy, ethical, and trustworthy as they relate to dairy products, but consumers used the terms distinctly. Consumers perceived differences in these terms between general categories of dairy as well as among products in a specific dairy category. In the second study, an online survey with three MaxDiff exercises was conducted to determine the importance of 63 label claims to consumer definitions of the terms sustainable, natural, and healthy. Claims related to animal welfare and happiness or simple, minimal ingredients and processing were considered by consumers to be important for all three terms. Priming consumers with information did not impact consumer definitions of any of these terms. -

Economic and Trade Agreement Between the Government of the United States of America and the Government of the People’S Republic of China
ECONOMIC AND TRADE AGREEMENT BETWEEN THE GOVERNMENT OF THE UNITED STATES OF AMERICA AND THE GOVERNMENT OF THE PEOPLE’S REPUBLIC OF CHINA PREAMBLE The Government of the United States of America and the Government of the People’s Republic of China (collectively the “Parties”), RECOGNIZING the importance of their bilateral economic and trade relationship; REALIZING that it is in the interests of both countries that trade grow and that there is adherence to international norms so as to promote market-based outcomes; CONVINCED of the benefits of contributing to the harmonious development and expansion of world trade and providing a catalyst to broader international cooperation; ACKNOWLEDGING the existing trade and investment concerns that have been identified by the Parties; and RECOGNIZING the desirability of resolving existing and any future trade and investment concerns as constructively and expeditiously as possible, HAVE AGREED as follows: CHAPTER 1 INTELLECTUAL PROPERTY Section A: General Obligations The United States recognizes the importance of intellectual property protection. China recognizes the importance of establishing and implementing a comprehensive legal system of intellectual property protection and enforcement as it transforms from a major intellectual property consumer to a major intellectual property producer. China believes that enhancing intellectual property protection and enforcement is in the interest of building an innovative country, growing innovation-driven enterprises, and promoting high quality economic growth. Article 1.1: China and the United States hereby affirm that they undertake provisions with respect to intellectual property, as set forth in Sections A through K. Article 1.2: The Parties shall ensure fair, adequate, and effective protection and enforcement of intellectual property rights. -

Process for Manufacturing Cheese Using Milk Protein Concentrate Powder
(19) TZZ ¥_T (11) EP 2 647 293 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 09.10.2013 Bulletin 2013/41 A23C 9/00 (2006.01) A23C 9/13 (2006.01) A23C 9/142 (2006.01) A23C 9/18 (2006.01) (2006.01) (2006.01) (21) Application number: 13175158.8 A23C 19/00 A23C 19/05 A23J 1/20 (2006.01) A23C 9/152 (2006.01) (22) Date of filing: 10.06.2009 (84) Designated Contracting States: • O’Kennedy, Brendan T AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Carlow (IE) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • Kelly, Philip M PT RO SE SI SK TR Carlow (IE) • Mounsey, John (30) Priority: 10.06.2008 IE 20080476 Carlow (IE) (62) Document number(s) of the earlier application(s) in (74) Representative: Gates, Marie Christina Esther et al accordance with Art. 76 EPC: Tomkins & Co. 09761746.8 / 2 309 869 5 Dartmouth Road Dublin 6 (IE) (71) Applicant: TEAGASC, The Agriculture and Food Development Remarks: Authority This application was filed on 04-07-2013 as a Dublin 4 (IE) divisional application to the application mentioned under INID code 62. (72) Inventors: • Guinee, Timothy P Carlow (IE) (54) Process for manufacturing cheese using milk protein concentrate powder (57) The present invention relates to the develop- to the use of the MCCa and MPCCa powders in the prep- ment of micellar sasin (MCCa) and milk protein concen- aration of reassembled milks. More specifically the in- trate (MPCCa) powders, with different calcium- to-casein vention relates to the use of such reassembled milks in ratios and buffering capacity. -
Ultrafiltration and Reverse Osmosis in Dairy Technology: a Review
801 Journal of Food Protection, Vol. 54, No. 10, Pages 801-809 (October 1991) Copyright© International Association of Milk, Food and Environmental Sanitarians Ultrafiltration and Reverse Osmosis in Dairy Technology: A Review FATHY E. EL-GAZZAR and ELMER H. MARTH* Department of Food Science and The Food Research Institute, University of Wisconsin-Madison, Madison, Wisconsin 53706 Downloaded from http://meridian.allenpress.com/jfp/article-pdf/54/10/801/1661973/0362-028x-54_10_801.pdf by guest on 24 September 2021 (Received for publication January 11, 1991) ABSTRACT no higher than 35°C. These conditions impose limitations on cleaning procedures that can be used industrially. Be Ultrafiltration and reverse osmosis processes can be useful in cause of these limitations cellulose acetate has now been the dairy foods industry. When milk is processed, milk fat and superseded by other polymers more tolerant to pH and casein are rejected fully (e.g., are in retentate) and thus are temperature. Polyamides were used for some time since concentrated by ultrafiltration and reverse osmosis membranes. they are better able to handle higher temperatures and a Lactic cultures are slow to reduce the pH of retentate because of wider pH range than is cellulose acetate. Polysulfones, its increased buffering capacity since concentrated calcium phos which are now frequently used, are a better alternative phate and proteins are present. Conditions for growth of patho genic microorganisms and inhibition of such bacteria in ultrafiltered because they have excellent hydrogen-bonding capabilities milk differ from those of unfiltered milk. The principal advantage and are very strong and rigid when wet.