Commonwealth of the Northern Mariana Islands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Register of Historic Places Registration Form This Form Is for Use in Nominating Or Requesting Determinations for Individual Properties and Districts
NPS Form 10-900 OMB No. 1024-0018 United States Department of the Interior National Park Service National Register of Historic Places Registration Form This form is for use in nominating or requesting determinations for individual properties and districts. See instructions in National Register Bulletin, How to Complete the National Register of Historic Places Registration Form. If any item does not apply to the property being documented, enter "N/A" for "not applicable." For functions, architectural classification, materials, and areas of significance, enter only categories and subcategories from the instructions. 1. Name of Property Historic name: _Hotel Honokaa Club Other names/site number: Honokaa Club Hotel, Honokaa Club, TMK: (3) 4-5-006:013 Name of related multiple property listing: Historic and Architectural Resources of Honokaʻa Town, Hawaiʻi Island, Hawaiʻi (Enter "N/A" if property is not part of a multiple property listing 2. Location Street & number: 45-3480 Māmane Street City or town: _Honokaʻa State: _HI County: _Hawaiʻi Not For Publication: Vicinity: 3. State/Federal Agency Certification As the designated authority under the National Historic Preservation Act, as amended, I hereby certify that this nomination request for determination of eligibility meets the documentation standards for registering properties in the National Register of Historic Places and meets the procedural and professional requirements set forth in 36 CFR Part 60. In my opinion, the property meets does not meet the National Register Criteria. I recommend that this property be considered significant at the following level(s) of significance: national _X statewide local Applicable National Register Criteria: _X A B _X C D Signature of certifying official/Title: Date State or Federal agency/bureau or Tribal Government In my opinion, the property meets does not meet the National Register criteria. -

Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas
Terrestrial Arthropod Surveys on Pagan Island, Northern Marianas Neal L. Evenhuis, Lucius G. Eldredge, Keith T. Arakaki, Darcy Oishi, Janis N. Garcia & William P. Haines Pacific Biological Survey, Bishop Museum, Honolulu, Hawaii 96817 Final Report November 2010 Prepared for: U.S. Fish and Wildlife Service, Pacific Islands Fish & Wildlife Office Honolulu, Hawaii Evenhuis et al. — Pagan Island Arthropod Survey 2 BISHOP MUSEUM The State Museum of Natural and Cultural History 1525 Bernice Street Honolulu, Hawai’i 96817–2704, USA Copyright© 2010 Bishop Museum All Rights Reserved Printed in the United States of America Contribution No. 2010-015 to the Pacific Biological Survey Evenhuis et al. — Pagan Island Arthropod Survey 3 TABLE OF CONTENTS Executive Summary ......................................................................................................... 5 Background ..................................................................................................................... 7 General History .............................................................................................................. 10 Previous Expeditions to Pagan Surveying Terrestrial Arthropods ................................ 12 Current Survey and List of Collecting Sites .................................................................. 18 Sampling Methods ......................................................................................................... 25 Survey Results .............................................................................................................. -

2006 XTERRA Saipan Press Guide.Qxd
AND THE SAIPAN SPORTS FEST MARCH 31 - APRIL 9 2006 PRESS GUIDE ssppoonnssoorrss The 2006 XTERRA Saipan Championship is presented by the Marianas Visitors Authority, and sponsored by the Pacific Islands Club, Paul Mitchell, XTERRA Gear, and Coca~Cola Beverage Co. (Micronesia), Inc. iinnttoo tthhee jjuunnggllee . AT THE 5th ANNUAL XTERRA SAIPAN CHAMPIONSHIP XTERRA is the world’s premier off-road multisport event. An extreme competition, the XTERRA format combines swimming with mountain biking and trail running. It has been described as one part triathlon, one part mountain bike race, and one part “survival of the fittest”. With its white sand beaches, warm water, jungle trails, and secret caves it’s easy to see how the XTERRA Saipan Championship became the “crown jewel” of the XTERRA Global Tour. In 2006 there will be more than 100 XTERRA races in 17 countries…but none quite like this one! The race starts with a 1.5-kilometer swim in the crystal clear waters fronting Micro Beach, follows with a 30k mountain bike ride that traverses island terrain to the top of Mount Tapotchau (the highest point on the island at 1,545 feet elevation) and finishes with a 12k trail run that takes competitors past World War II relics and through secret jungles and caves. The event is a qualifier for the 2006 Nissan Xterra World Championship held in Maui on October 29 - with 34 slots available to the top finishers in each age group (see page 12 for details). The race will also award points to amateur athletes racing in this year’s Nissan Xterra USA Championship Series. -

Title 90-30 Vendor Site Regulations
TITLE 90: MARIANAS VISITORS AUTHORITY CHAPTER 90-30 VENDOR SITE REGULATIONS Part 001 General Provisions § 90-30-305 Display of Permit § 90-30-001 Purposes § 90-30-310 Occupation of Area § 90-30-005 Authority and Effect § 90-30-315 Sign § 90-30-010 Severability § 90-30-320 Solicitation § 90-30-015 Definitions § 90-30-325 Cleanliness and Orderliness Part 100 Designated Vendor Sites § 90-30-330 Vendor Employees § 90-30-101 Vendor Sites § 90-30-335 Destruction of Trees and Improvements Part 200 Permits § 90-30-340 Parking § 90-30-201 Permit Required § 90-30-345 Other Laws § 90-30-205 Application Forms § 90-30-350 Amplified Sound § 90-30-210 Fees § 90-30-355 Permit Requirements § 90-30-215 Accompanying Documents and Information Part 400 Penalties § 90-30-220 Designation of Vendor § 90-30-401 Suspension; Conditions Sites of Reinstatement § 90-30-225 Permit Categories § 90-30-405 Hearings and Appeals § 90-30-230 Permit Decision Appendix A Saipan Part 300 Miscellaneous Appendix B Tinian § 90-30-301 Structures Appendix C Rota Chapter Authority: 4 CMC § 2128. Chapter History: Adopted 21 Com. Reg. 16799 (May 19, 1999); Proposed 21** Com. Reg. 16461 (Feb. 18, 1999); Amdts Proposed 18 Com. Reg. 14819 (Dec. 15, 1996);* Amdts Emergency 18 Com. Reg. 14147 (June 15, 1996) (effective for 120 days from June 6, 1996); Amdts Adopted 14 Com. Reg. 10363 (Dec. 15, 1992); Amdts Proposed 14 Com. Reg. 9974 (Oct. 15, 1992); Amdts Certified 14 Com. Reg. 8655 (Jan. 15, 1992); Amdts Adopted 12 Com. Reg. 7146 (June 15, 1990); Amdts Proposed 12 Com. -

Congressional Record—House
January 23, 2012 CONGRESSIONAL RECORD — HOUSE H87 Schneider National, ran one of the Na- ROTA CULTURAL AND NATURAL Northern Mariana Islands, as a unit of the tion’s largest trucking companies with RESOURCES STUDY ACT National Park System; and (2) consider management alternatives for over 12,500 tractors, 35,000 trailers, and Mr. WITTMAN. Madam Speaker, I thousands and thousands of employees. the island of Rota, Commonwealth of the move to suspend the rules and pass the Northern Mariana Islands. Some of you may recognize those bill (H.R. 1141) to authorize the Sec- (b) STUDY PROCESS AND COMPLETION.—Ex- trucks painted in a distinct shade of retary of the Interior to study the suit- cept as provided by subsection (c) of this sec- orange that travel the highways and ability and feasibility of designating tion, section 8(c) of Public Law 91–383 (16 byways of America. prehistoric, historic, and limestone for- U.S.C. 1a–5(c)) shall apply to the conduct and Mr. Schneider was a hardworking est sites on Rota, Commonwealth of completion of the study required by this sec- tion. man who began his career driving a the Northern Mariana Islands, as a truck and as a mechanic’s assistant at (c) SUBMISSION OF STUDY RESULTS.—Not unit of the National Park System. later than 3 years after the date that funds age 18 in his family’s business. He The Clerk read the title of the bill. are made available for this section, the Sec- served in Korea, went to the Wharton The text of the bill is as follows: retary shall submit to the Committee on School of Business in Philadelphia, and H.R. -

Marianas Variety' ^ Micronesia’S Leading Newspaper Since 1972
¿/Marianas Variety' ^ Micronesia’s Leading Newspaper Since 1972 Vol. 20-No. 62 Sopn, MP 96950 © 1991 Marianas Variety October 18. 1991 Sewing CNMI for 19 Years 50 Ruling on linian case questionable--AG by Rafael H. Arroyo nor Lorenzo I. DL. Guerrero regarding the scope and extent to be reviewed by the highest parameters on how the initiative dated September 23, 1991, At of the authority granted to the court of the land,” Naraja said in process works.. The Office of the Attorney torney General Robert Naraja commission. his memorandum. Such a definitive ruling is also General has determined that its described the decision penned The trial court decision in up According to Naraja’s analy seen to clear all doubts on such filing of an appeal before the by erstwhile Chief Judge Robert holding the supremacy of a local sis of the issue, only a decision things as the priority of laws; the Supreme Court on the case it A. Hefner as “contrary to appli law over Commonwealth-wide from the CNMI Supreme Court scope and extent of legislation brought against the Tinian Ca cable law” in either the CNMI or law allegedly ignored at least can establish legal precedent to which may be enacted through sino Gaming Commission is other jurisdictions. 100 years of state court decisions secure once and for all a defini the initiative process; the posi- necessary, saying that the ruling He branded Hefner’s declara from other US jurisdictions, tive ruling on whether the initia C ontinued on page 24 issued out by the Superior Court tory judgment on the case as claiming that the CNMI consti tive is above Commonwealth- was “extremely questionable.” “not definitive,” such that there tution is different than other wide laws. -

1999 Number 02 Feb 18
COMMONWEALTH OF THE NORTHERN MARIANA ISLANDS SAIPAN MARIANA ISLANDS VOLUME 21 NUMBER 02 FEBRUARY 18,1999 COMMONWEALTH REGISTER COMMONWEALTH REGISTER VOLUME 21 NUMBER 02 FEBRUARY 18,1999 TABLE OF CONTENTS PROPOSED and AMENDMENTS: Proposed Permanent Amendment to the Personnel Service System Rules & Regs. Civil Service Commission. ...................................................................... -16455 Marianas Visitors Authority Vendor Site Regulations. Marianas Visitors Authority. ...................................................................... 16461 Administrative Plan for Rental Assistance Program. Northern Marianas Housing Corporation....................................................... .I6471 Grievance Procedures for Section 8 Program. Northern Marianas Housing Corporation.. ..................................................... .I6534 Entrance Fee to the Commonwealth Museum of History & Culture. Northern Mariana Islands Museum.. ............................................................ .I6534 NOTICE OF ADOPTION: Adoption of Amendments of the Health Screening Requirements of Alien Employees Regulations. Commonwealth Health Center. .....................................................................I6554 Adoption of Proposed New Section I. 10 of the Excepted Service Personnel Regulations. Office of Personnel Management.. ...............................................................-16559 Adoption of the Amended CRM Jet Ski Rules and Regulations. Coastal Resources Management. ................................................................ -

The Attitudes of Japanese Tourists Towards Cultural Heritage Attractions in the Cnmi
MICRONESIAN JOURNAL OF THE HUMANITIES AND SOCIAL SCIENCES Vol. 5, nº 1/2 Combined Issue November 2006 THE ATTITUDES OF JAPANESE TOURISTS TOWARDS CULTURAL HERITAGE ATTRACTIONS IN THE CNMI Elizabeth Sayers National Parks Association of Queensland Inc Dirk HR Spennemann Institute for Land, Water and Society, Charles Sturt University Eco-tourism and cultural tourism is being regarded as a major strategy to open up new tourism markets for some Micronesian states. With Japanese visitors making up the single largest contingent of tourists to Micronesia, there is a need to examine their attitudes towards the cultural and historical elements of the islands. Are they in fact interested in such attributes and what are their expectations for a visit? This paper examines the attitudes and expectations of a co- hort of Japanese tourists to the CNMI and outlines the challenge faced by the cultural heritage and tourism management authorities. Tourism has been regarded as the panacea to nese (Data: Guam Visitors Bureau; Marianas cure the economic troubles of the Pacific Visitors Authority; Palau Visitor Authority). Island communities, particularly of the Pacific Given the size of the market on the one microstates. Mass tourism, particularly from hand, and the desire of the regional tourism Japan, has long reached Guam (Page & Lawton development agencies to develop cultural and 1996; Iversen 1997), but also, more recently, eco-tourism opportunities (Heather et al 2000; Saipan and Palau (Page & Lawton 1996). The Look & Spennemann 2000; Spennemann et al Japanese tourist market is one of the largest in 2001) it is surprising that information on the contemporary Micronesia. -

Draft Programmatic Environmental Impact Statement for the Non-Contiguous United States
Nationwide Public Safety Broadband Network Draft Programmatic Environmental Impact Statement for the Non-Contiguous United States First Responder Network Authority Volume 5 - Chapter 7 NORTHERN MARIANA ISLANDS Alaska Hawaii American Samoa Guam Northern Mariana Islands Puerto Rico U.S. Virgin Islands March 2016 -Page Intentionally Left Blank- First Responder Network Authority Nationwide Public Safety Broadband Network Draft Programmatic Environmental Impact Statement for the Non-Contiguous United States Volume 5 Amanda Goebel Pereira, AICP NEPA Coordinator First Responder Network Authority U.S. Department of Commerce 12201 Sunrise Valley Dr. M/S 243 Reston, VA 20192 Cooperating Agencies Federal Communications Commission General Services Administration U.S. Department of Agriculture—Rural Utilities Service U.S. Department of Agriculture—U.S. Forest Service U.S. Department of Agriculture—Natural Resource Conservation Service U.S. Department of Defense—Department of the Air Force U.S. Department of Energy U.S. Department of Homeland Security March 2016 Cover Art Sources: Map Service. 2015. OpenStreetMap. ArcGIS Map Image Layer by Esri. Sourced from: Esri, HERE, DeLorme, TomTom, Intermap, increment P Corp., GEBCO, USGS, FAO, NPS, NRCAN, GeoBase, IGN, Kadaster NL, Ordnance Survey, Esri Japan, METI, Esri China (Hong Kong), swisstopo, MapmyIndia, © OpenStreetMap contributors, and the GIS User Community. NOAA (National Oceanic and Atmospheric Administration). 2016. Polar bear (Ursus maritimus). Uncredited Marine Mammal Commission Photograph. Accessed: January 2016. Retrieved from: http://search.noaa.gov/search/images?utf8=%E2%9C%93&sc=0&query=Polar+bear+%28Ursus+maritimus%29&m=&affiliate=noaa. gov&commit=Search NPS (National Park Service). 2016. Fruit Bat [White-necked Flying Fox (Pteropus tonganus)]. Uncredited NPS Photograph. -

Congressional Record—House H3663
June 17, 2013 CONGRESSIONAL RECORD — HOUSE H3663 area a much safer and pleasant experi- and the Rota bridled white-eye birds, that the Secretary of the Interior to deter- ence on Y Mountain, and so I urge my are also native to the island of Rota. Three mine whether it would be suitable and colleagues to vote for this bill. endangered plant species are also found on feasible to add certain cultural, ar- I yield back the balance of my time. Rota and two are endemic to the island. cheological, historical, and natural re- (7) Because of the significant cultural and The SPEAKER pro tempore. The natural resources listed above, on September sources on the island of Rota in the question is on the motion offered by 2005, the National Park Service, Pacific West Northern Marianas to the National the gentleman from Utah (Mr. BISHOP) Region, completed a preliminary resource Park System. that the House suspend the rules and assessment on the island of Rota, Common- The House has already voted to au- pass the bill, H.R. 253, as amended. wealth of the Northern Mariana Islands, thorize the suitability and feasibility The question was taken. which determined that the ‘‘establishment of study for Rota on two separate occa- The SPEAKER pro tempore. In the a unit of the national park system sions, but the other body did not follow opinion of the Chair, two-thirds being appear[ed] to be the best way to ensure the long term protection of Rota’s most impor- through, so here we are again. -

Northern Mariana Islands
provider of tax revenues for the government, which also had to face mounting social problems. An esti- mated two thousand businesses closed down, teachers’ contracts were not renewed, and many other public ser- vices had to be scaled back. By year’s end, there was even serious talk of reducing work hours for government employees. Meanwhile, the legislature passed several measures affecting the presence of guest workers, and a seemingly coordinated campaign to close the garment industry down resulted in a spate of negative media publicity. This resulted in increased pressure for Congress to adopt legisla- tion to federalize immigration and the minimum wage, and even the customs territory of the Commonwealth. Governor Teno inherited a severely depleted treasury. The new adminis- tration found that the tax-rebate holding account (holding withheld tax money for eventual rebate to the tax- payers), which was supposed to con- tain $34 million, contained only $2 million. The missing $32 million has never been identified. In addition, the Northern Mariana Islands public auditor charged that the Mari- anas Visitors Bureau had misspent up Despite a more conciliatory and less to $12 million in illegal contracts, and confrontational administration in that the governor (Lang) had spent Saipan, relations between the Com- unappropriated millions on sole- monwealth of the Northern Mariana source contracts. The governor was Islands (cnmi) and Washington also accused of lending substantial continued to deteriorate. Third-term sums to the Tinian Casino Gaming governor Pedro P Tenorio (“Teno”) Commission to meet their salaries, inherited a major fiscal quagmire and to pay a “consultant” a total of from his predecessor, Froilan C Teno- $730,000 for four years plus benefits. -
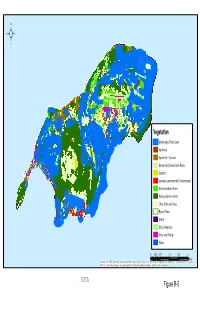
Rota Figure R-6 ²
² Vegetation Undeveloped Public Land Agroforest Agroforest -- Coconut Barren/Sandy Beach/Bare Rocks Cropland Leucaena Leucocephala (Tangantangan) Mixed Introduced Forest Native Limestone Forest Other Shrub and Grass Ravine Forest Strand Urban Vegetation Urban and Built-up Water 0 0.375 0.75 1.5 2.25 3 Miles Sources: Esri, HERE, DeLorme, Intermap, increment P Corp., GEBCO, USGS, FAO, NPS, NRCAN, GeoBase, IGN, Kadaster NL, Ordnance Survey, Esri Japan, METI, Esri China (Hong Kong), swisstopo, MapmyIndia, © OpenStreetMap contributors, and the GIS User Community ² Mochong Rota Latte Stone Quarry Commissioner's OfficeRectory Suitability Map I Chenchon Park Sabanan Heights Nanyo Kohatsu Kabushiki Kaisha Sugar Mill Japanese Hospital Fish Reserve Wedding Cake Mountain Nat'l Register of Historic Places Japanese Coastal Defense Gun Critical Habitat Mariana Crow Mariana Crow - Fruitbat - G-M Kingfisher Undeveloped >= 1 HA. 10% Slope 1.5 0.75 0 1.5 Miles Sources: Esri, HERE, DeLorme, Intermap, increment P Corp., GEBCO, USGS, FAO, NPS, NRCAN, GeoBase, IGN, Kadaster NL, Ordnance Survey, Esri Japan, METI, Esri China (Hong Kong), swisstopo, MapmyIndia, © OpenStreetMap contributors, and the GIS User Community ROTA FIGURE R-6 ² Future Public Land Use Power Plant Village Homesteads Agricultural Homesteads Civic Uses Solar Farm Undeveloped >= 1 HA. 10% Slope 1 0.5 0 1 Miles Sources: Esri, HERE, DeLorme, Intermap, increment P Corp., GEBCO, USGS, FAO, NPS, NRCAN, GeoBase, IGN, Kadaster NL, Ordnance Survey, Esri Japan, METI, Esri China (Hong Kong), swisstopo,