Print Annual Reviews for Fiscal Year 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Phenylbutyrate (PB), Is a New Active Substance
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion for the approval of Ammonpas. This scientific discussion has been updated until 1 November 2001. For information on changes after this date please refer to module 8B. 1. Introduction Ammonaps, sodium phenylbutyrate (PB), is a new active substance. It is indicated as adjunctive therapy in the chronic management of urea cycle disorders, involving deficiencies of carbamylphosphate synthetase, ornithine transcarbamylase, or argininosuccinate synthetase. It is indicated in all patients with neonatal-onset presentation (complete enzyme deficiencies, presenting within the first 28 days of life). It is also indicated in patients with late-onset disease (partial enzyme deficiencies, presenting after the first month of life) who have a history of hyperammonaemic encephalopathy. The dossier submitted in support of the application comprises data generated by the applicant: all chemical/pharmaceutical data, the two mutagenicity studies for Part III, and for Part IV, the bioequivalence study and a review of the US IND/NDA programme. Additional information was available from published literature. Urea cycle disorders (UCD) are inherited deficiencies of one of the enzymes involved in the urea cycle, by which ammonium is converted to urea. Ammonium is highly toxic to nerve cells and hyperammonaemia may result in metabolic derangement, leading to anorexia, lethargy, confusion, coma, brain damage, and death. The most severe forms of UCDs occur early in life (complete enzyme deficiencies). The classic neonatal presentation of all the UCD (with the exception of arginase deficiency) is quite uniform and includes, after a short symptom-free interval of one to five days, poor feeding, vomiting, lethargy, muscular hypotonia, hyperventilation, irritability and convulsions. -
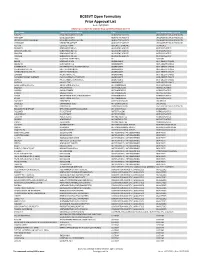
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

Product List March 2019 - Page 1 of 53
Wessex has been sourcing and supplying active substances to medicine manufacturers since its incorporation in 1994. We supply from known, trusted partners working to full cGMP and with full regulatory support. Please contact us for details of the following products. Product CAS No. ( R)-2-Methyl-CBS-oxazaborolidine 112022-83-0 (-) (1R) Menthyl Chloroformate 14602-86-9 (+)-Sotalol Hydrochloride 959-24-0 (2R)-2-[(4-Ethyl-2, 3-dioxopiperazinyl) carbonylamino]-2-phenylacetic 63422-71-9 acid (2R)-2-[(4-Ethyl-2-3-dioxopiperazinyl) carbonylamino]-2-(4- 62893-24-7 hydroxyphenyl) acetic acid (r)-(+)-α-Lipoic Acid 1200-22-2 (S)-1-(2-Chloroacetyl) pyrrolidine-2-carbonitrile 207557-35-5 1,1'-Carbonyl diimidazole 530-62-1 1,3-Cyclohexanedione 504-02-9 1-[2-amino-1-(4-methoxyphenyl) ethyl] cyclohexanol acetate 839705-03-2 1-[2-Amino-1-(4-methoxyphenyl) ethyl] cyclohexanol Hydrochloride 130198-05-9 1-[Cyano-(4-methoxyphenyl) methyl] cyclohexanol 93413-76-4 1-Chloroethyl-4-nitrophenyl carbonate 101623-69-2 2-(2-Aminothiazol-4-yl) acetic acid Hydrochloride 66659-20-9 2-(4-Nitrophenyl)ethanamine Hydrochloride 29968-78-3 2,4 Dichlorobenzyl Alcohol (2,4 DCBA) 1777-82-8 2,6-Dichlorophenol 87-65-0 2.6 Diamino Pyridine 136-40-3 2-Aminoheptane Sulfate 6411-75-2 2-Ethylhexanoyl Chloride 760-67-8 2-Ethylhexyl Chloroformate 24468-13-1 2-Isopropyl-4-(N-methylaminomethyl) thiazole Hydrochloride 908591-25-3 4,4,4-Trifluoro-1-(4-methylphenyl)-1,3-butane dione 720-94-5 4,5,6,7-Tetrahydrothieno[3,2,c] pyridine Hydrochloride 28783-41-7 4-Chloro-N-methyl-piperidine 5570-77-4 -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

WO 2017/173059 Al 5 October 2017 (05.10.2017) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2017/173059 Al 5 October 2017 (05.10.2017) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, C12N 9/26 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KH, KN, (21) International Application Number: KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, PCT/US20 17/024981 MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, (22) International Filing Date: NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, 30 March 2017 (30.03.2017) RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, (25) Filing Language: English ZA, ZM, ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 62/3 15,400 30 March 2016 (30.03.2016) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/457,584 10 February 2017 (10.02.2017) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, 15/473,994 30 March 2017 (30.03.2017) US TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (71) Applicant: AMICUS THERAPEUTICS, INC. -

2018-2019 Targeted Medication Safety Best Practices for Hospitals
2018-2019 Targeted Medication Safety Best Practices for Hospitals The purpose of the Targeted Medication Safety Best Practices for Hospitals is to identify, inspire, and mobilize widespread, national adoption of consensus-based best practices for specific medication safety issues that continue to cause fatal and harmful errors in patients, despite repeated warnings in ISMP publications. Hospitals can focus their medication safety efforts over the next 2 years on these best practices, which are realistic and have been successfully adopted by numerous organizations. While targeted for the hospital-based setting, some best practices may be applicable to other healthcare settings. The Targeted Medication Safety Best Practices for Hospitals have been reviewed by an external expert advisory panel and approved by the ISMP Board of Trustees. Related issues of the ISMP Medication Safety Alert! are referenced after each best practice. ISMP encourages hospitals that have not implemented the 2016-2017 Targeted Medication Safety Best Practices for Hospitals to do so as a priority, while implementing the 2018-2019 best practices. Organizations need to focus on previous best practices 2, 3, 9 and 11 since these have the lowest implementation rate. Two of the 2016-2017 Targeted Medication Safety Best Practices for Hospitals (number 4 and 7) have been revised for 2018-2019. Best practices number 12 through 14 are new for 2018-2019. www.ismp.org BEST PRACTICE 1: Dispense vinCRIStine (and other vinca alkaloids) in a minibag of a compatible solution and not in a syringe. Rationale: Related ISMP Medication The goal of this best practice is to ensure that vinca alkaloids are Safety Alerts!: administered by the intravenous route only. -

WHO Drug Information Vol 22, No
WHO Drug Information Vol 22, No. 1, 2008 World Health Organization WHO Drug Information Contents Challenges in Biotherapeutics Miglustat: withdrawal by manufacturer 21 Regulatory pathways for biosimilar Voluntary withdrawal of clobutinol cough products 3 syrup 22 Pharmacovigilance Focus Current Topics WHO Programme for International Drug Proposed harmonized requirements: Monitoring: annual meeting 6 licensing vaccines in the Americas 23 Sixteen types of counterfeit artesunate Safety and Efficacy Issues circulating in South-east Asia 24 Eastern Mediterranean Ministers tackle Recall of heparin products extended 10 high medicines prices 24 Contaminated heparin products recalled 10 DacartTM development terminated and LapdapTM recalled 11 ATC/DDD Classification Varenicline and suicide attempts 11 ATC/DDD Classification (temporary) 26 Norelgestromin-ethynil estradiol: infarction ATC/DDD Classification (final) 28 and thromboembolism 12 Emerging cardiovascular concerns with Consultation Document rosiglitazone 12 Disclosure of transdermal patches 13 International Pharmacopoeia Statement on safety of HPV vaccine 13 Cycloserine 30 IVIG: myocardial infarction, stroke and Cycloserine capsules 33 thrombosis 14 Erythropoietins: lower haemoglobin levels 15 Recent Publications, Erythropoietin-stimulating agents 15 Pregabalin: hypersensitivity reactions 16 Information and Events Cefepime: increased mortality? 16 Assessing the quality of herbal medicines: Mycophenolic acid: pregnancy loss and contaminants and residues 36 congenital malformation 17 Launch -

Enzyme Replacement Therapy Srx-0019 Policy Type ☒ Medical ☐ Administrative ☐ Payment
MEDICAL POLICY STATEMENT Original Effective Date Next Annual Review Date Last Review / Revision Date 06/15/2011 03/15/2017 10/04/2016 Policy Name Policy Number Enzyme Replacement Therapy SRx-0019 Policy Type ☒ Medical ☐ Administrative ☐ Payment Medical Policy Statements prepared by CSMG Co. and its affiliates (including CareSource) are derived from literature based on and supported by clinical guidelines, nationally recognized utilization and technology assessment guidelines, other medical management industry standards, and published MCO clinical policy guidelines. Medically necessary services include, but are not limited to, those health care services or supplies that are proper and necessary for the diagnosis or treatment of disease, illness, or injury and without which the patient can be expected to suffer prolonged, increased or new morbidity, impairment of function, dysfunction of a body organ or part, or significant pain and discomfort. These services meet the standards of good medical practice in the local area, are the lowest cost alternative, and are not provided mainly for the convenience of the member or provider. Medically necessary services also include those services defined in any Evidence of Coverage documents, Medical Policy Statements, Provider Manuals, Member Handbooks, and/or other policies and procedures. Medical Policy Statements prepared by CSMG Co. and its affiliates (including CareSource) do not ensure an authorization or payment of services. Please refer to the plan contract (often referred to as the Evidence of Coverage) for the service(s) referenced in the Medical Policy Statement. If there is a conflict between the Medical Policy Statement and the plan contract (i.e., Evidence of Coverage), then the plan contract (i.e., Evidence of Coverage) will be the controlling document used to make the determination. -

BCBSVT Specialty Drug List Effective 2021.07.01.Xlsx
Effective Date: 07/01/2021 SPECIALTY DRUG LIST Revised Date: 05/07/2021 DOSAGE EXCLUDED ON NATIONAL DRUG CLASS DRUG NAME GENERIC NAME FORM PERFORMANCE FORMULARY ANEMIA ARANESP SOLN DARBEPOETIN ALFA SOLN INJ ANEMIA ARANESP SOSY DARBEPOETIN ALFA SOLN PREFILLED SYRINGE ANEMIA EPOGEN SOLN EPOETIN ALFA INJ X ANEMIA PROCRIT SOLN EPOETIN ALFA INJ X ANEMIA REBLOZYL SOLR LUSPATERCEPT-AAMT FOR SUBCUTANEOUS INJ ANEMIA RETACRIT SOLN EPOETIN ALFA-EPBX INJ ANTI-GOUT AGENT KRYSTEXXA SOLN PEGLOTICASE INJ (FOR IV INFUSION) ANTI-INFECTIVE PREVYMIS SOLN LETERMOVIR IV SOLN ANTI-INFECTIVE PREVYMIS TABS LETERMOVIR TAB ASTHMA CINQAIR SOLN RESLIZUMAB IV INFUSION SOLN ASTHMA FASENRA SOSY BENRALIZUMAB SUBCUTANEOUS SOLN PREFILLED SYRINGE ASTHMA FASENRA PEN SOAJ BENRALIZUMAB SUBCUTANEOUS SOLN AUTO-INJECTOR ASTHMA NUCALA SOAJ MEPOLIZUMAB SUBCUTANEOUS SOLUTION AUTO-INJECTOR ASTHMA NUCALA SOLR MEPOLIZUMAB FOR INJ ASTHMA NUCALA SOSY MEPOLIZUMAB SUBCUTANEOUS SOLUTION PREF SYRINGE ASTHMA XOLAIR SOLR OMALIZUMAB FOR INJ ASTHMA XOLAIR SOSY OMALIZUMAB SUBCUTANEOUS SOLN PREFILLED SYRINGE CARDIOVASCULAR VYNDAMAX CAPS TAFAMIDIS CAP CARDIOVASCULAR VYNDAQEL CAPS TAFAMIDIS MEGLUMINE (CARDIAC) CAP CENTRAL NERVOUS SYSTEM AGENTS AUSTEDO TABS DEUTETRABENAZINE TAB CENTRAL NERVOUS SYSTEM AGENTS ENSPRYNG SOSY SATRALIZUMAB-MWGE SUBCUTANEOUS SOLN PREF SYRINGE CENTRAL NERVOUS SYSTEM AGENTS HETLIOZ CAPS TASIMELTEON CAPSULE CENTRAL NERVOUS SYSTEM AGENTS HETLIOZ LQ SUSP TASIMELTEON ORAL SUSP CHEMOTHERAPY PROTECTANT AMIFOSTINE SOLR AMIFOSTINE CRYSTALLINE FOR INJ CHEMOTHERAPY PROTECTANT ELITEK -

Horizon Therapeutics Public Annual Report 2020
Horizon Therapeutics Public Annual Report 2020 Form 10-K (NASDAQ:HZNP) Published: February 26th, 2020 PDF generated by stocklight.com octb inte UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2019 or ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number 001-35238 HORIZON THERAPEUTICS PUBLIC LIMITED COMPANY (Exact name of Registrant as specified in its charter) Ireland Not Applicable (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) Connaught House, 1st Floor 1 Burlington Road, Dublin 4, D04 C5Y6, Ireland Not Applicable (Address of principal executive offices) (Zip Code) 011 353 1 772 2100 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of Each Class Trading Symbol Name of Each Exchange on Which Registered Ordinary shares, nominal value $0.0001 per share HZNP The Nasdaq Global Select Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐. Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒. Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

The Effectiveness of Correcting Abnormal Metabolic Profiles
Received: 25 April 2019 Revised: 17 June 2019 Accepted: 19 June 2019 DOI: 10.1002/jimd.12139 ORIGINAL ARTICLE The effectiveness of correcting abnormal metabolic profiles Peter Theodore Clayton UCL Great Ormond Street Institute of Child Health, London, UK Abstract Inborn errors of metabolism cause disease because of accumulation of a metabolite Correspondence before the blocked step or deficiency of an essential metabolite downstream of the Peter Clayton, UCL Great Ormond Street Institute of Child Health, London, UK. block. Treatments can be directed at reducing the levels of a toxic metabolite or Email: [email protected] correcting a metabolite deficiency. Many disorders have been treated successfully first in a single patient because we can measure the metabolites and adjust treat- Communicating Editor: Sander M. Houten ment to get them as close as possible to the normal range. Examples are drawn from Komrower's description of treatment of homocystinuria and the author's trials 5 of treatment in bile acid synthesis disorders (3β-hydroxy-Δ -C27-steroid dehydro- genase deficiency and Δ4-3-oxosteroid 5β-reductase deficiency), neurotransmitter amine disorders (aromatic L-amino acid decarboxylase [AADC] and tyrosine hydroxylase deficiencies), and vitamin B6 disorders (pyridox(am)ine phosphate oxidase deficiency and pyridoxine-dependent epilepsy [ALDH7A1 deficiency]). Sometimes follow-up shows there are milder and more severe forms of the disease and even variable clinical manifestations but by measuring the metabolites we can adjust the treatment to get the metabolites into the normal range. Biochemical mea- surements are not subject to placebo effects and will also show if the disorder is improving spontaneously. -

Express Scripts 2020 National Preferred Formulary List
2020 Express Scripts National Preferred Formulary List The 2020 National Preferred Formulary drug list is shown below. The formulary is the list of drugs included in your prescription plan. Inclusion on the list does not guarantee coverage. The following list is not a complete list of over-the-counter [OTC] products and prescription medical supplies that are on the formulary. The only OTC products and prescription medical supplies that appear on the list are in contracted classes. PLEASE NOTE: Brand-name drugs may move to nonformulary status if a generic version becomes available during the year. Not all the drugs listed are covered by all prescription plans; check your benefit materials for the specific drugs covered and the copayments for your prescription plan. For specific questions about your coverage, please call the phone number printed on your member ID card. KEY For the member: FDA-approved generic medications meet strict standards and [INJ] – Injectable Drug contain the same active ingredients as their corresponding brand-name medications, [OTC] – Over-the-counter Product although they may have a different appearance. [SP] – Specialty Drug For the physician: Please prescribe preferred products and allow generic Brand-name drugs are listed in CAPITAL letters. Example: ABILIFY MAINTENA substitutions when medically appropriate. Generic drugs are listed in lower-case letters. Example: ibuprofen A adenosine [INJ] amabelz anthralin ADVAIR HFA amantadine APLISOL [INJ] abacavir [SP] ADVATE [INJ] [SP] AMBISOME [INJ] APOKYN [INJ] [SP]