In Danish Wastewater Treatment Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novel Bacterial Lineages Associated with Boreal Moss Species Hannah
bioRxiv preprint doi: https://doi.org/10.1101/219659; this version posted November 16, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Novel bacterial lineages associated with boreal moss species 2 Hannah Holland-Moritz1,2*, Julia Stuart3, Lily R. Lewis4, Samantha Miller3, Michelle C. Mack3, Stuart 3 F. McDaniel4, Noah Fierer1,2* 4 Affiliations: 5 1Cooperative Institute for Research in Environmental Sciences, University of Colorado at Boulder, 6 Boulder, CO, USA 7 2Department of Ecology and Evolutionary Biology, University of Colorado at Boulder, Boulder, CO, 8 USA 9 3Center for Ecosystem Science and Society, Northern Arizona University, Flagstaff, AZ USA 10 4Department of Biology, University of Florida, Gainesville, FL 32611-8525, USA 11 *Corresponding Author 12 13 Abstract 14 Mosses are critical components of boreal ecosystems where they typically account for a large 15 proportion of net primary productivity and harbor diverse bacterial communities that can be the major 16 source of biologically-fixed nitrogen in these ecosystems. Despite their ecological importance, we have 17 limited understanding of how microbial communities vary across boreal moss species and the extent to 18 which local environmental conditions may influence the composition of these bacterial communities. 19 We used marker gene sequencing to analyze bacterial communities associated with eight boreal moss 20 species collected near Fairbanks, AK USA. We found that host identity was more important than site in 21 determining bacterial community composition and that mosses harbor diverse lineages of potential N2- 22 fixers as well as an abundance of novel taxa assigned to understudied bacterial phyla (including 23 candidate phylum WPS-2). -

Edaphobacter Dinghuensis Sp. Nov., an Acidobacterium Isolated from Lower Subtropical Forest Soil Jia Wang,3 Mei-Hong Chen,3 Ying-Ying Lv, Ya-Wen Jiang and Li-Hong Qiu
International Journal of Systematic and Evolutionary Microbiology (2016), 66, 276–282 DOI 10.1099/ijsem.0.000710 Edaphobacter dinghuensis sp. nov., an acidobacterium isolated from lower subtropical forest soil Jia Wang,3 Mei-hong Chen,3 Ying-ying Lv, Ya-wen Jiang and Li-hong Qiu Correspondence State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University, Li-hong Qiu Guangzhou 510275, PR China [email protected] An aerobic bacterium, designated DHF9T, was isolated from a soil sample collected from the lower subtropical forest of Dinghushan Biosphere Reserve, Guangdong Province, PR China. Cells were Gram-stain-negative, non-motile, short rods that multiplied by binary division. Strain DHF9T was an obligately acidophilic, mesophilic bacterium capable of growth at pH 3.5–5.5 (optimum pH 4.0) and at 10–33 8C (optimum 28–33 8C). Growth was inhibited at NaCl concentrations above 2.0 % (w/v). The major fatty acids were iso-C15 : 0,C16 : 0 and C16 : 1v7c. The polar lipids consist of phosphatidylethanolamine, two unidentified aminolipids, two unidentified phospholipids, two unidentified polar lipids and an unidentified glycolipid. The DNA G+C content was 57.7 mol%. Phylogenetic analysis based on 16S rRNA gene sequences indicated that the strain belongs to the genus Edaphobacter in subdivision 1 of the phylum Acidobacteria, with the highest 16S rRNA gene sequence similarity of 97.0 % to Edaphobacter modestus Jbg-1T. Based on phylogenetic, chemotaxonomic and physiological analyses, it is proposed that strain DHF9T represents a novel species of the genus Edaphobacter, named Edaphobacter dinghuensis sp. nov. -

Genomic Analysis of Family UBA6911 (Group 18 Acidobacteria)
bioRxiv preprint doi: https://doi.org/10.1101/2021.04.09.439258; this version posted April 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 2 Genomic analysis of family UBA6911 (Group 18 3 Acidobacteria) expands the metabolic capacities of the 4 phylum and highlights adaptations to terrestrial habitats. 5 6 Archana Yadav1, Jenna C. Borrelli1, Mostafa S. Elshahed1, and Noha H. Youssef1* 7 8 1Department of Microbiology and Molecular Genetics, Oklahoma State University, Stillwater, 9 OK 10 *Correspondence: Noha H. Youssef: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2021.04.09.439258; this version posted April 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 11 Abstract 12 Approaches for recovering and analyzing genomes belonging to novel, hitherto unexplored 13 bacterial lineages have provided invaluable insights into the metabolic capabilities and 14 ecological roles of yet-uncultured taxa. The phylum Acidobacteria is one of the most prevalent 15 and ecologically successful lineages on earth yet, currently, multiple lineages within this phylum 16 remain unexplored. Here, we utilize genomes recovered from Zodletone spring, an anaerobic 17 sulfide and sulfur-rich spring in southwestern Oklahoma, as well as from multiple disparate soil 18 and non-soil habitats, to examine the metabolic capabilities and ecological role of members of 19 the family UBA6911 (group18) Acidobacteria. -

(Phaseolus Vulgaris) in Native and Agricultural Soils from Colombia Juan E
Pérez-Jaramillo et al. Microbiome (2019) 7:114 https://doi.org/10.1186/s40168-019-0727-1 RESEARCH Open Access Deciphering rhizosphere microbiome assembly of wild and modern common bean (Phaseolus vulgaris) in native and agricultural soils from Colombia Juan E. Pérez-Jaramillo1,2,3, Mattias de Hollander1, Camilo A. Ramírez3, Rodrigo Mendes4, Jos M. Raaijmakers1,2* and Víctor J. Carrión1,2 Abstract Background: Modern crop varieties are typically cultivated in agriculturally well-managed soils far from the centers of origin of their wild relatives. How this habitat expansion impacted plant microbiome assembly is not well understood. Results: Here, we investigated if the transition from a native to an agricultural soil affected rhizobacterial community assembly of wild and modern common bean (Phaseolus vulgaris) and if this led to a depletion of rhizobacterial diversity. The impact of the bean genotype on rhizobacterial assembly was more prominent in the agricultural soil than in the native soil. Although only 113 operational taxonomic units (OTUs) out of a total of 15,925 were shared by all eight bean accessions grown in native and agricultural soils, this core microbiome represented a large fraction (25.9%) of all sequence reads. More OTUs were exclusively found in the rhizosphere of common bean in the agricultural soil as compared to the native soil and in the rhizosphere of modern bean accessions as compared to wild accessions. Co-occurrence analyses further showed a reduction in complexity of the interactions in the bean rhizosphere microbiome in the agricultural soil as compared to the native soil. Conclusions: Collectively, these results suggest that habitat expansion of common bean from its native soil environment to an agricultural context had an unexpected overall positive effect on rhizobacterial diversity and led to a stronger bean genotype-dependent effect on rhizosphere microbiome assembly. -

Microbial Network, Phylogenetic Diversity and Community Membership in the Active Layer Across a Permafrost Thaw Gradient
Environmental Microbiology (2017) 19(8), 3201–3218 doi:10.1111/1462-2920.13809 Microbial network, phylogenetic diversity and community membership in the active layer across a permafrost thaw gradient Rhiannon Mondav ,1,2* Carmody K. McCalley ,3,4† disturbance affecting habitat filtering. Hydrogenotro- Suzanne B. Hodgkins ,5 Steve Frolking ,4 phic methanogens and Acidobacteria dominated the Scott R. Saleska ,3 Virginia I. Rich ,6‡ bog shifting from palsa-like to fen-like at the water- Jeff P. Chanton 5 and Patrick M. Crill 7 line. The fen (no underlying permafrost, high 1Department of Ecology and Genetics, Limnology, radiative forcing signature) had the highest alpha, Uppsala University, Uppsala 75236, Sweden. beta and phylogenetic diversity, was dominated by 2School of Chemistry and Molecular Biosciences, Proteobacteria and Euryarchaeota and was signifi- University of Queensland, Brisbane, QLD 4072, cantly enriched in methanogens. The Mire microbial Australia. network was modular with module cores consisting 3Department of Ecology and Evolutionary Biology, of clusters of Acidobacteria, Euryarchaeota or Xan- University of Arizona, Tucson, AZ 85721, USA. thomonodales. Loss of underlying permafrost with 4Institute for the Study of Earth, Oceans, and Space, associated hydrological shifts correlated to changes University of New Hampshire, Durham, NH 03824, in microbial composition, alpha, beta and phyloge- USA. netic diversity associated with a higher radiative 5Department of Earth Ocean and Atmospheric Science, forcing signature. These results support the complex role of microbial interactions in mediating carbon Florida State University, Tallahassee, FL 32306-4320, budget changes and climate feedback in response to USA. climate forcing. 6Department of Soil, Water and Environmental Science, University of Arizona, Tucson, AZ 85721, USA. -

Tree-Aggregated Predictive Modeling of Microbiome Data
www.nature.com/scientificreports OPEN Tree‑aggregated predictive modeling of microbiome data Jacob Bien1, Xiaohan Yan2, Léo Simpson3,4 & Christian L. Müller4,5,6* Modern high‑throughput sequencing technologies provide low‑cost microbiome survey data across all habitats of life at unprecedented scale. At the most granular level, the primary data consist of sparse counts of amplicon sequence variants or operational taxonomic units that are associated with taxonomic and phylogenetic group information. In this contribution, we leverage the hierarchical structure of amplicon data and propose a data‑driven and scalable tree‑guided aggregation framework to associate microbial subcompositions with response variables of interest. The excess number of zero or low count measurements at the read level forces traditional microbiome data analysis workfows to remove rare sequencing variants or group them by a fxed taxonomic rank, such as genus or phylum, or by phylogenetic similarity. By contrast, our framework, which we call trac (tree‑aggregation of compositional data), learns data‑adaptive taxon aggregation levels for predictive modeling, greatly reducing the need for user‑defned aggregation in preprocessing while simultaneously integrating seamlessly into the compositional data analysis framework. We illustrate the versatility of our framework in the context of large‑scale regression problems in human gut, soil, and marine microbial ecosystems. We posit that the inferred aggregation levels provide highly interpretable taxon groupings that can help microbiome researchers gain insights into the structure and functioning of the underlying ecosystem of interest. Microbial communities populate all major environments on earth and signifcantly contribute to the total plan- etary biomass. Current estimates suggest that a typical human-associated microbiome consists of ∼ 1013 bacteria1 and that marine bacteria and protists contribute to as much as 70% of the total marine biomass2. -

Microbial Communities of Polymetallic Deposits' Acidic
Microbial Communities of Polymetallic Deposits’ Acidic Ecosystems of ANGOR UNIVERSITY Continental Climatic Zone With High Temperature Contrasts Gavrilov, Sergei N.; Korzhenkov, Aleksei A.; Kublanov, Ilya V.; Bargiela, Rafael; Zamana, Leonid V.; Toshchakov, Stepan V.; Golyshin, Peter; Golyshina, Olga Frontiers in Microbiology PRIFYSGOL BANGOR / B Published: 17/07/2019 Publisher's PDF, also known as Version of record Cyswllt i'r cyhoeddiad / Link to publication Dyfyniad o'r fersiwn a gyhoeddwyd / Citation for published version (APA): Gavrilov, S. N., Korzhenkov, A. A., Kublanov, I. V., Bargiela, R., Zamana, L. V., Toshchakov, S. V., Golyshin, P., & Golyshina, O. (2019). Microbial Communities of Polymetallic Deposits’ Acidic Ecosystems of Continental Climatic Zone With High Temperature Contrasts. Frontiers in Microbiology. Hawliau Cyffredinol / General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. 01. Oct. 2021 ORIGINAL RESEARCH published: 17 July 2019 doi: 10.3389/fmicb.2019.01573 Microbial Communities of Polymetallic Deposits’ Acidic Ecosystems of Continental Climatic Zone With High Temperature Contrasts Sergey N. -
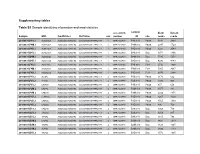
Supplementary Tables Table S1 Sample Identifying Information And
Supplementary tables Table S1 Sample identifying information and read statistics accession sample #raw #clean Sample MID FwdPrimer RvPrimer run number ID site reads reads 20100831.P4S.1 ACACGAGA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 P4S.910 Palsa 3831 2893 20100831.P4M.1 ACATAGCA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 P4M.910 Palsa 2047 1729 20100831.P4D.1 ACGATACA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 P4D.910 Palsa 3221 2543 20100831.B4S.1 ACTGCTCA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 B4S.910 Bog 1971 1366 20100831.B4M.1 AGCAGCGC ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 B4M.910 Bog 5530 5251 20100831.B4D.1 AGCGTGCA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 B4D.910 Bog 4296 4143 20100831.F4S.1 AGTCTAGC ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 F4S.910 Fen* 2716 1890 20100831.F4M.1 ATGACTGA ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 F4M.910 Fen* 5005 3987 20100831.F4D.1 ATGCACGC ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 1 SRR1023739 F4D.910 Fen* 2870 2384 20100901.P1S.2 ACTGAT ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 2 SRR1023740 P1S.910 Palsa 1170 662 20100901.P2S.2 ATGTGT ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 2 SRR1023740 P2S.910 Palsa 1320 923 20100901.P3S.2 CACAGT ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 2 SRR1023740 P3S.910 Palsa 857 656 20100901.P2M.2 CACTAC ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 2 SRR1023740 P2M.910 Palsa 1077 881 20100901.P3M.2 CAGCAT ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT 2 SRR1023740 P3M.910 Palsa 2032 1474 20100901.P1D.2 CTAGAGC ACGGGCGGTGWGTRC CCGYCAATTCMTTTRAGTTT -

Pyrosequencing Investigation Into the Bacterial Community in Permafrost Soils Along the China-Russia Crude Oil Pipeline (CRCOP)
Pyrosequencing Investigation into the Bacterial Community in Permafrost Soils along the China-Russia Crude Oil Pipeline (CRCOP) Sizhong Yang1*, Xi Wen2, Huijun Jin1, Qingbai Wu1 1 State Key Laboratory of Frozen Soil Engineering (SKLFSE), Cold and Arid Regions Environmental and Engineering Research Institute (CAREERI), Chinese Academy of Sciences, Lanzhou, China, 2 College of Electrical Engineering, Northwest University for Nationalities, Lanzhou, China Abstract The China-Russia Crude Oil Pipeline (CRCOP) goes through 441 km permafrost soils in northeastern China. The bioremediation in case of oil spills is a major concern. So far, little is known about the indigenous bacteria inhabiting in the permafrost soils along the pipeline. A pilot 454 pyrosequencing analysis on the communities from four selected sites which possess high environment risk along the CRCOP is herein presented. The results reveal an immense bacterial diversity than previously anticipated. A total of 14448 OTUs with 84834 reads are identified, which could be assigned into 39 different phyla, and 223 families or 386 genera. Only five phyla sustain a mean OTU abundance more than 5% in all the samples, but they altogether account for 85.08% of total reads. Proteobacteria accounts for 41.65% of the total OTUs or 45% of the reads across all samples, and its proportion generally increases with soil depth, but OTUs numerically decline. Among Proteobacteria, the abundance of Beta-, Alpha-, Delta- and Gamma- subdivisions average to 38.7% (2331 OTUs), 37.5% (2257 OTUs), 10.35% (616 OTUs), and 6.21% (374 OTUs), respectively. Acidobacteria (esp. Acidobacteriaceae), Actinobacteria (esp. Intrasporangiaceae), Bacteroidetes (esp. Sphingobacteria and Flavobacteria) and Chloroflexi (esp. -

The Effect of Tillage System and Crop Rotation on Soil Microbial Diversity and Composition in a Subtropical Acrisol
Diversity 2012, 4, 375-395; doi:10.3390/d4040375 OPEN ACCESS diversity ISSN 1424-2818 www.mdpi.com/journal/diversity Article The Effect of Tillage System and Crop Rotation on Soil Microbial Diversity and Composition in a Subtropical Acrisol Patricia Dorr de Quadros 1,2,*, Kateryna Zhalnina 2, Austin Davis-Richardson 2, Jennie R. Fagen 2, Jennifer Drew 2, Cimelio Bayer 1, Flavio A.O. Camargo 1 and Eric W. Triplett 2 1 Department of Soil Science, Federal University of Rio Grande do Sul, Porto Alegre, Brazil; E-Mails: [email protected] (C.B.); [email protected] (F.A.O.C.) 2 Department of Microbiology and Cell Science, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, FL, USA; E-Mails: [email protected] (K.Z.), [email protected] (A.C.R.), [email protected] (J.D.), [email protected] (E.W.T.) *Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: (352)-575-5731. Received: 29 August 2012; in revised form: 11 October 2012 / Accepted: 19 October 2012 / Published: 31 October 2012 Abstract: Agricultural management alters physical and chemical soil properties, which directly affects microbial life strategies and community composition. The microbial community drives important nutrient cycling processes that can influence soil quality, cropping productivity and environmental sustainability. In this research, a long-term agricultural experiment in a subtropical Acrisol was studied in south Brazil. The plots at this site represent two tillage systems, two nitrogen fertilization regimes and three crop rotation systems. Using Illumina high-throughput sequencing of the 16S rRNA gene, the archaeal and bacterial composition was determined from phylum to species level in the different plot treatments. -

The First Representative of the Globally Widespread Subdivision 6 Acidobacteria, Vicinamibacter Silvestris Gen
International Journal of Systematic and Evolutionary Microbiology (2016), 66, 2971–2979 DOI 10.1099/ijsem.0.001131 The first representative of the globally widespread subdivision 6 Acidobacteria, Vicinamibacter silvestris gen. nov., sp. nov., isolated from subtropical savannah soil Katharina J. Huber,1 Alicia M. Geppert,1 Gerhard Wanner,2 Barbel€ U. Fösel,1 Pia K. Wüst1 and Jörg Overmann1,3 Correspondence 1Department of Microbial Ecology and Diversity Research, Leibniz Institute DSMZ – German Katharina J. Huber Collection of Microorganisms and Cell Cultures, Braunschweig, Germany [email protected] 2Department of Biology I, Biozentrum Ludwig Maximilian University of Munich, Planegg- Martinsried, Germany 3Technical University Braunschweig, Braunschweig, Germany Members of the phylum Acidobacteria are abundant in a wide variety of soil environments. Despite this, previous cultivation attempts have frequently failed to retrieve representative phylotypes of Acidobacteria, which have, therefore, been discovered by culture-independent methods (13175 acidobacterial sequences in the SILVA database version 123; NR99) and only 47 species have been described so far. Strain Ac_5_C6T represents the first isolate of the globally widespread and abundant subdivision 6 Acidobacteria and is described in the present study. Cells of strain Ac_5_C6T were Gram-stain-negative, immotile rods that divided by binary fission. They formed yellow, extremely cohesive colonies and stable aggregates even in rapidly shaken liquid cultures. Ac_5_C6T was tolerant of a wide range of temperatures (12–40 C) and pH values (4.7–9.0). It grew chemoorganoheterotrophically on a broad range of substrates including different sugars, organic acids, nucleic acids and complex proteinaceous compounds. T The major fatty acids of Ac_5_C6 were iso-C17 : 1 !9c,C18 : 1 !7c and iso-C15 : 0. -

Influence of Resistance-Inducing Chemical Elicitors Against Pine Wilt
microorganisms Article Influence of Resistance-Inducing Chemical Elicitors against Pine Wilt Disease on the Rhizosphere Microbiome 1, 1, 2 3 1 Mohamed Mannaa y , Gil Han y, Hee Won Jeon , Junheon Kim , Namgyu Kim , Ae Ran Park 2 , Jin-Cheol Kim 2,* and Young-Su Seo 1,* 1 Department of Integrated Biological Science, Pusan National University, Busan 46241, Korea; [email protected] (M.M.); [email protected] (G.H.); [email protected] (N.K.) 2 Division of Applied Bioscience and Biotechnology, Chonnam National University, Gwangju 61186, Korea; [email protected] (H.W.J.); [email protected] (A.R.P.) 3 Forest Insect Pests and Diseases Division, National Institute of Forest Science, Seoul 02455, Korea; [email protected] * Correspondence: [email protected] (J.-C.K.); [email protected] (Y.-S.S.) These authors contributed equally to this work. y Received: 20 May 2020; Accepted: 10 June 2020; Published: 11 June 2020 Abstract: Pine wilt disease (PWD) caused by Bursaphelenchus xylophilus is a major threat to pine forests worldwide. Induction of resistance is a promising and safe management option that should be investigated in relation to its possible influence on the pine tree ecosystem, including the surrounding microbial communities. In this study, two main resistance-inducing chemical elicitors, methyl salicylic acid (MeSA) and acibenzolar-s-methyl (ASM), were tested for their control efficiency against PWD and their influence on the rhizosphere microbial composition. Foliar treatment of pine seedlings with the chemical elicitors resulted in a reduction in PWD severity, with ASM showing better control efficacy, reaching up to 73% compared to the untreated control.