Phytoplankton Diversity Related to Pollution from Mula River at Pune City
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Review of Water Pollution of Pawana River
© 2018 JETIR July 2018, Volume 5, Issue 7 www.jetir.org (ISSN-2349-5162) COMPARATIVE REVIEW OF WATER POLLUTION OF PAWANA RIVER BHARAT D. GIDDE ABSTRACT-Pune , one of the metropolitan city of India , with population approximately 70 lacs .The city has two municipal corporations Pune Municipal Corporation (PMC) and PimpriChinchwad Municipal Corporation (PCMC) . Pune and PimpriChinchwad are the twin cities and well connected by rail and most popular asindustrial,educationaland auto hub of our country.ThePawana river originates in western ghats near Lonawala flowing towards south-East and flows through PimpriChinchwad city covering a stretch of24 Km .Since the population of the city is increasing rapidly , it results in increase in domestic and other type of waste and it becomes a source of water pollution . The present study deals with the review of comparison of major physio-chemical parameters like DO , BOD and COD .The sample of river water collected for a period of three months May2016, August 2016 and January 2017and the analysis is done . Key words :---physio-chemical, Pawana river INTRODUCTION We always says “water is life “ but nobody wants to do any activity which preserves the water .Wasting of water is become a part of lifestyle .Water is used for various purposes by public . The water is used in large quantity by city water supply, industrialsectors ,and other similar agencies .The used water appears as a waste water .It may be treated before its final disposal .But because of loose legislations in our country at some sites the untreated effluent is directly discharged into the nerebywater bodies or river which pollutes the river water .The same thing is happening with Pawana river .The Pawana river flows through flows though PimpriChinchwad city and only source of city water supply as well as industrial water supply .The river enters in the city at village ravet . -

City Development Plan Pune Cantonment Board Jnnurm
City Development Plan Pune Cantonment Board JnNURM DRAFT REPORT, NOVEMBER 2013 CREATIONS ENGINEER’S PRIVATE LIMITED City Development Plan – Pune Cantonment Board JnNURM Abbreviations WORDS ARV Annual Rental Value CDP City Development Plan CEO Chief Executive Officer CIP City Investment Plan CPHEEO Central Public Health and Environmental Engineering Organisation FOP Financial Operating Plan JNNURM Jawaharlal Nehru National Urban Renewal Mission KDMC Kalyan‐Dombivali Municipal Corporation LBT Local Body Tax MoUD Ministry of Urban Development MSW Municipal Solid Waste O&M Operation and Maintenance PCB Pune Cantonment Board PCMC Pimpri‐Chinchwad Municipal Corporation PCNTDA Pimpri‐Chinchwad New Town Development Authority PMC Pune Municipal Corporation PMPML Pune MahanagarParivahanMahamandal Limited PPP Public Private Partnership SLB Service Level Benchmarks STP Sewerage Treatment Plant SWM Solid Waste Management WTP Water Treatment Plant UNITS 2 Draft Final Report City Development Plan – Pune Cantonment Board JnNURM Km Kilometer KW Kilo Watt LPCD Liter Per Capita Per Day M Meter MM Millimeter MLD Million Litres Per Day Rmt Running Meter Rs Rupees Sq. Km Square Kilometer Tn Tonne 3 Draft Final Report City Development Plan – Pune Cantonment Board JnNURM Contents ABBREVIATIONS .................................................................................................................................... 2 LIST OF TABLES ..................................................................................................................................... -

Download Document
94 SEAC-3 Day 01 SEAC Meeting number: 94 Meeting Date September 23, 2019 Subject: Environment Clearance for Mula, Mutha,Mula-Mutha River Rejuvenation Project Is a Violation Case: No 1.Name of Project Mula, Mutha,Mula-Mutha River Rejuvenation Project 2.Type of institution Government 3.Name of Project Proponent Pune Municipal Corporation 4.Name of Consultant Green Circle Inc. 5.Type of project River Rejuvenation Project 6.New project/expansion in existing project/modernization/diversification Not applicable in existing project 7.If expansion/diversification, whether environmental clearance Not applicable has been obtained for existing project 8.Location of the project Pune 9.Taluka Pune 10.Village Pune Correspondence Name: Mr. Mangesh Dighe Room Number: NA Floor: NA Building Name: PMC Building Road/Street Name: NA Locality: Shivajinagar City: Pune 11.Whether in Corporation / Municipal Municipal / other area NA 12.IOD/IOA/Concession/Plan IOD/IOA/Concession/Plan Approval Number: NA Approval Number Approved Built-up Area: 00 13.Note on the initiated work (If NA applicable) 14.LOI / NOC / IOD from MHADA/ NA Other approvals (If applicable) 15.Total Plot Area (sq. m.) 820 ha 16.Deductions NA 17.Net Plot area 820 Ha a) FSI area (sq. m.): 00 18 (a).Proposed Built-up Area (FSI & b) Non FSI area (sq. m.): 00 Non-FSI) c) Total BUA area (sq. m.): 00 Approved FSI area (sq. m.): 00 18 (b).Approved SEAC-AGENDA-0000000329Built up area as per Approved Non FSI area (sq. m.): 00 DCR Date of Approval: 23-03-2018 19.Total ground coverage (m2) 8200000 20.Ground-coverage Percentage (%) (Note: Percentage of plot not open 8200000 to sky) 21.Estimated cost of the project 28000000000 22.Number of buildings & its configuration Joy S.Thakur (Secretary SEAC Meeting No: 94 Meeting Date: September Page 1 of Shri. -
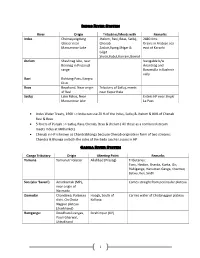
1 Indus River System River Origin Tributries/Meets with Remarks
Indus River System River Origin Tributries/Meets with Remarks Indus Chemayungdung Jhelum, Ravi, Beas, Satluj, 2880 Kms Glacier near Chenab Drains in Arabian sea Mansarovar Lake Zaskar,Syang,Shigar & east of Karachi Gilgit Shyok,Kabul,Kurram,Gomal Jhelum Sheshnag lake, near Navigable b/w Beninag in Pirpanjal Anantnag and range Baramulla in Kashmir vally Ravi Rohtang Pass, Kangra Distt. Beas Beaskund, Near origin Tributary of Satluj, meets of Ravi near Kapurthala Satluj Lake Rakas, Near Enters HP near Shipki Mansarovar lake La Pass Indus Water Treaty, 1960 :-> India can use 20 % of the Indus, Satluj & Jhelum & 80% of Chenab Ravi & Beas 5 Rivers of Punjab :-> Satluj, Ravi, Chenab, Beas & Jhelum ( All these as a combined stream meets Indus at Mithankot) Chenab in HP is known as Chandrabhanga because Chenab originate in form of two streams: Chandra & Bhanga on both the sides of the Bada Laccha La pass in HP. Ganga River System Ganga Tributary Origin Meeting Point Remarks Yamuna Yamunotri Glaciar Allahbad (Prayag) Tributaries: Tons, Hindon, Sharda, Kunta, Gir, Rishiganga, Hanuman Ganga, Chambal, Betwa, Ken, Sindh Son (aka ‘Savan’) Amarkantak (MP), Comes straight from peninsular plateau near origin of Narmada Damodar Chandawa, Palamau Hoogli, South of Carries water of Chotanagpur plateau distt. On Chota Kolkata Nagpur plateau (Jharkhand) Ramganga: Doodhatoli ranges, Ibrahimpur (UP) Pauri Gharwal, Uttrakhand 1 Gandak Nhubine Himal Glacier, Sonepur, Bihar It originates as ‘Kali Gandak’ Tibet-Mustang border Called ‘Narayani’ in Nepal nepal Bhuri Gandak Bisambharpur, West Khagaria, Bihar Champaran district Bhagmati Where three headwater streams converge at Bāghdwār above the southern edge of the Shivapuri Hills about 15 km northeast of Kathmandu Kosi near Kursela in the Formed by three main streams: the Katihar district Tamur Koshi originating from Mt. -

Study of Water Quality Parameters of Mula-Mutha River at Pune, Maharashtra (India)
International Journal of Lakes and Rivers. ISSN 0973-4570 Volume 13, Number 1 (2020), pp. 95-103 © Research India Publications http://www.ripublication.com/ijlr.htm Study of Water Quality Parameters of Mula-Mutha River at Pune, Maharashtra (India) S.D. Jadhav1, M.S. Jadhav2 1Department of Basic Sciences & Humanities, Bharati Vidyapeeth (Deemed To Be University), College of Engineering, Pune 411043, Maharashtra, India., 2 Department of Civil Engineering, Sinhgad Technical Education Society’s Sou., Venutai Chavan Polytechnic, Pune, Maharashtra, India. Abstract Water is one of the most important compounds in the world. The contamination and pollution of water is of great concern in the world for the developing countries like India. The question of water pollution has acquired a critical stage. Human activities, industries, hospitals, sewage water, agricultural diffused pollution are some of the sources of water pollution. Drinking water quality is one of the important environmental health detriments. Use of safe drinking water is a foundation for the control and prevention of water born diseases. In this work we have analyzed Mula-Mutha river water quality for drinking purpose. Various river water parameters were analyzed and are compared to established standards given by WHO. The river water analysis showed that the river water is not suitable for potable use in city area. Keywords: Mula-Mutha River, Pune City, Physico-chemical analysis, Water pollution INTRODUCTION The entire Pune City is covered by Mula-Mutha rivers. River Mula originates from Mulshi Dam which forms Mulshi Lake. River Mutha originates from Panshet Dam Via Khadakwasla Dam. It also flows through city of Pune and meets to river Mula at Shivajinagar area of Pune city, to that part it called as Sangam Bridge. -

Study of Sulphate Concentration of Pavana River at Pune
International Journal of Chemical Studies 2017; 5(2): 198-200 P-ISSN: 2349–8528 E-ISSN: 2321–4902 IJCS 2017; 5(2): 198-200 Study of sulphate concentration of Pavana River © 2017 JEZS Received: 02-01-2017 at Pune: A case study Accepted: 03-02-2017 SD Jadhav SD Jadhav and MS Jadhav Bharati Vidyapeeth Deemed University, College of Engineering, Pune, India. Abstract Pavana River in Pune (India) is one of the important water bodies to pollution because of the role played MS Jadhav by the river in carrying municipal and industrial wastes and run-off from agricultural lands in their basin. Sinhgad Technical Education In spite of various rules and regulations various small scale industries are discharging their wastes Society’s Sou. Venutai Chavan directly into the river body. The present investigation focus on study of sulphate concentration of Pavana Polytechnic, Pune, India. River water for the year 2015-16. For this study eight sampling stations were selected on the course of river. The results obtained were compared with the standard desirable limit values of drinking water given by WHO. The present study shed light on the quality of river water samples for various usages of the river water to all. Keywords: Pavana River, Sulphate concentration, agricultural run-off, agrochemicals 1. Introduction Rivers are the systems of surface water which carry the entire load of materials in the dissolved and undissolved things from both natural as well as anthropogenic sources in one direction only [1]. Sewage waters, Hospitals, Human activities, and use of various agrochemicals are some of the major factors which influence surface water quality [2, 3]. -

Biodiversity of Freshwater Mitosporic Fungi from Dhule District (M.S.), India S
Journal of Ecobiotechnology 2/6: 25-28, 2010 ISSN 2077-0464 http://journal-ecobiotechnology.com/ Biodiversity of Freshwater Mitosporic Fungi from Dhule District (M.S.), India S. Y. Patil1*, V. R. Patil2 and L. C. Nemade2 1P. G. Department of Botany, S.S.V.P.S.L.Kr.Dr.P.R. Ghogrey, Science College, Dhule (M.S.), India 2Shri V. S. Naik Arts, Commerce & Science College, Raver District- Jalgaon. 425508 (M.S.), India *Corresponding author, Email: [email protected] Keywords Abstract Five species belonging to two genera of Hyphomycetes were isolated from foam Freshwater fungi samples and submerged leaves collected from the river Panzara in Dhule district. Ingoldian Hyphomycetes Flabellospora acuminata Descals and Webster is being reported for the first time from North Maharashtra India. While Flabellospora crassa Alasoadura and Flabellospora multiradiata Nawawi are being reported for the first from Maharashtra state. 1. Introduction Freshwater hyphomycetes were practically moist condition in polythene bags. They were untouched until the pioneering work of Ingold washed several times in tap water and finally in (1942), who recognized them as ‘Aquatic distilled water. The selected leaves from each site Hyphomycetes’. Later these fungi have also been were cut into small bits and incubated separately in described as “Freshwater Hyphomycetes” (Nilsson, Petri dishes containing distilled water at laboratory 1964) and “Water borne Hyphomycetes” (Webster temperature (25-30°C). The water in the Petri and Descals, 1979). There are more than 500 dishes was replaced once in two days to minimize named species of hyphomycetes known from the growth of bacteria and other organisms. The freshwater habitats. -
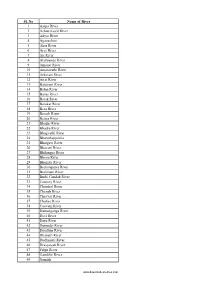
List of Rivers in India
Sl. No Name of River 1 Aarpa River 2 Achan Kovil River 3 Adyar River 4 Aganashini 5 Ahar River 6 Ajay River 7 Aji River 8 Alaknanda River 9 Amanat River 10 Amaravathi River 11 Arkavati River 12 Atrai River 13 Baitarani River 14 Balan River 15 Banas River 16 Barak River 17 Barakar River 18 Beas River 19 Berach River 20 Betwa River 21 Bhadar River 22 Bhadra River 23 Bhagirathi River 24 Bharathappuzha 25 Bhargavi River 26 Bhavani River 27 Bhilangna River 28 Bhima River 29 Bhugdoi River 30 Brahmaputra River 31 Brahmani River 32 Burhi Gandak River 33 Cauvery River 34 Chambal River 35 Chenab River 36 Cheyyar River 37 Chaliya River 38 Coovum River 39 Damanganga River 40 Devi River 41 Daya River 42 Damodar River 43 Doodhna River 44 Dhansiri River 45 Dudhimati River 46 Dravyavati River 47 Falgu River 48 Gambhir River 49 Gandak www.downloadexcelfiles.com 50 Ganges River 51 Ganges River 52 Gayathripuzha 53 Ghaggar River 54 Ghaghara River 55 Ghataprabha 56 Girija River 57 Girna River 58 Godavari River 59 Gomti River 60 Gunjavni River 61 Halali River 62 Hoogli River 63 Hindon River 64 gursuti river 65 IB River 66 Indus River 67 Indravati River 68 Indrayani River 69 Jaldhaka 70 Jhelum River 71 Jayamangali River 72 Jambhira River 73 Kabini River 74 Kadalundi River 75 Kaagini River 76 Kali River- Gujarat 77 Kali River- Karnataka 78 Kali River- Uttarakhand 79 Kali River- Uttar Pradesh 80 Kali Sindh River 81 Kaliasote River 82 Karmanasha 83 Karban River 84 Kallada River 85 Kallayi River 86 Kalpathipuzha 87 Kameng River 88 Kanhan River 89 Kamla River 90 -

Physicochemical Analysis of Mula Mutha River Pune
Civil Engineering and Urban Planning:An International Journal(CiVEJ) Vol.2,No.2, June 2015 PHYSICOCHEMICAL ANALYSIS OF MULA MUTHA RIVER PUNE Pali Sahu 1, Sonali Karad 2, Sagar Chavan 2 and Sourabh Khandelwal 2 1Asst.Prof. Environmental Engineering, Civil Department, VIIT, Pune, India 2U.G. Student, Civil Department, VIIT, Pune, India ABSTRACT Mula-Mutha River in pune (India) is one of the most vulnerable water bodies to pollution because of their role in carrying municipal and industrial wastes and run-offs from agricultural lands in their vast drainage basins. Despite of the various standards and laws made by government many industries were discharging their waste directly into the river making its quality poor day by day. The restoration of river water quality has been a major challenge to the environmental managers. Detailed research and analysis is needed to evaluate different process and mechanism involved in polluting water. The aim of the work under the title is to analyze the river by dividing it into various sampling station. The present study also identifies the critical pollutants affecting the river water quality during its course through the city. The indices have been computed for pre-monsoon, monsoon and post-monsoon season at four locations, Khadakwasla , Sangamwadi, Vithalwadi &Bund Garden. It was found that the water quality ranged from satisfactory to marginal category at Khadakwasla and fell under very poor category at all other locations. This research have a vast future scope as the rapid industrialization results in formation of toxic contaminants leading to enormous damages to environment directly putting the lives at risk. -

2020090269.Pdf
INDEX Sr. No. Description Page No. Part I:District Survey Report for Sand Mining Or River Bed Mining 1. Introduction 8 2. Overview of Mining Activity in the District 8 3. The list of Mining Leases in the district with the location and period of Validity 10 4. Details of royalty or revenue received in last three year 11 5. Details Of Production Of Sand Or Bajri Or Minor Mineral In Last Three Years 11 6. Recommendation of Enforcement & Monitoring Guidelines for Sand Mining by 11 MoEF&CC- 2020: 7. Process Of Deposition Of Sediments In The Rivers Of The District 16 8. General profile of the district 24 9. Land Utilization Pattern Of The District 26 10. Physiography Of The District 27 11. Rainfall Data Of The District 28 12. Geology Of The District 29 Part II: for Other Minor Minerals other than sand 1 Introduction 39 2 Overview of mining activity in the district 39 3 General Profile of district 41 4 Geology of District 44 5 Drainage of Irrigation pattern; 45 6 Land Utilization Pattern in the District: Forest, Agricultural, Horticultural, Mining 45 etc. 7 Surface Water and Ground Water scenario of the district 47 8 Rainfall of the district and climatic condition 48 9 Details of the mining leases in the District as per the format 49 10 Details of Royalty or Revenue received in last three years 50 11 Details of Production of Minor Mineral in last three years 50 12 Mineral Map of the District 51 13 List of Letter of Intent (LOI) Holders in the District along with its validity as per 51 the format 14 Total Mineral Reserve available in the District 52 15 Quality /Grade of Mineral available in the District 52 16 Use of Mineral 52 17 Demand and Supply of the Mineral in the last three years 52 18 Mining leases marked on the map of the district 52 19 Details of the area of where there is a cluster of mining leases viz. -

Pollution Status of River Mula (Pune City) Maharashtra, India
J. Ecophysiol. Occup. Hlth. 11 (2011) 81-90 ©2011 The Academy of Environmental Biology, India Pollution status of river Mula (Pune city) Maharashtra, India A.D.Kshirsagar and V.R. Gunale Department of Botany, University of Pune, Pune – 411 007, (MS, India). Abstract : Present work deals with the seasonal variations in physico-chemical parameters of river Mula at Pune city. Water samples were collected monthly from selected sampling stations (Station I- Wakad; II- Aundh and III- Dapodi) in winter, summer and monsoon seasons during October 2007 to September 2008. The analysis was carried out for temperature, pH, dissolved oxygen (DO), free carbon dioxide (free CO2), total alkalinity, total hardness, biological oxygen demand (BOD), chemical oxygen demand (COD), chloride, nitrate and phosphate. It was observed that, temperature, chloride, BOD, COD, total alkalinity, total hardness, nitrate and phosphate content was high during summer than winter and less during monsoon seasons. Whereas, the DO content decreased in the summer and increased in the winter followed by monsoon seasons. The quality of water at station II and station III were high in term of nutrient loads, due to influent domestic wastewater. These results suggest that the water quality of river Mula is adversely affected and impaired by the discharge of domestic waste. Key Words: Mula river, Physico-chemical parameters, Water pollution. Introduction Numerous researchers have studied the physico-chemical parameters of various river According to the United Nation’s World Water water in the India. It has been found that the Development Report (2003), 70% of the earth’s water quality of the river lying in the vicinities of surface is covered by water; of which only 2.5% urban areas is heavily polluted due to industrial of water is fresh and only 0.3% water is and domestic wastes. -

SPECIES DIVERSITY of GENUS SCENEDESMUS MEYEN.FROM RIVER PANZARA of DHULE CITY MAHARASHTRA (INDIA) 1Kumavat M. R., 1Borse K. N
I J R B A T, Vol. VI (Special Issue 2), Feb 2018 : 204-207 e-ISSN 2347 – 517X INTERNATIONAL JOURNAL OF RESEARCHES IN BIOSCIENCES, AGRICULTURE AND TECHNOLOGY © VISHWASHANTI MULTIPURPOSE SOCIETY (Global Peace Multipurpose Society) R. No. MH-659/13(N) www.ijrbat.in SPECIES DIVERSITY OF GENUS SCENEDESMUS MEYEN.FROM RIVER PANZARA OF DHULE CITY MAHARASHTRA (INDIA) 1Kumavat M. R., 1Borse K. N., 2Pawar N. S. and 3Jain D. S. 1S. S. V. P. S.’s L. K. Dr. P. R. Ghogrey Science College, Dhule 435005 M.S. India 2S. S. V. P. S.’s Arts, Commerce and Science College, Shindkheda 425406 M.S. India 3Gangamai Arts , Comm., and Science College Nagaon Dist.-Dhule 424005 [email protected]; [email protected] ABSTRACT: Green algae are diverse and ubiquitous in aquatic habitat. Chlorococcales is an order of green algae in the classChlorophyceae .The members were unicellular, coenocyticor colonial the predominant phase in the life-history is a non-motile one. The genus Scenedesmus Meyen are free living planktonic mostly in a shallow confined water. While studying the algal flora of Panzara river authors came across several members of order Chlorococcales. The present paper deals with the systematic account of 13 taxa of genus ScenedesmusMeyen.S. muzzanensis Huber- Pestalozzi, S. perforates Lemmermiann Var. major (Turner) Comb. Nov., Scenedesmus perforates Lemmermiann, S. armatus (Chodat) G.M.Smith Var. longlariensis Hortob f. biaudatus Hortob, S. armatus (Chodat) G.M.Smith Var. longlariensis Hortob f. biaudatus Hortob, S. quadricauda (Turpin) Brebisson Var. quadrispina (Chodat) G. M. Smith, S. bijugatus( Turpin ) Kuetzing forma parvus (G.M.Smith)comb.