Eye-Based Interaction in Graphical Systems: Theory & Practice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -

Extraocular Muscles Orbital Muscles
EXTRAOCULAR MUSCLES ORBITAL MUSCLES INTRA- EXTRA- OCULAR OCULAR CILIARY MUSCLES INVOLUNTARY VOLUNTARY 1.Superior tarsal muscle. 1.Levator Palpebrae Superioris 2.Inferior tarsal muscle 2.Superior rectus 3.Inferior rectus 4.Medial rectus 5.Lateral rectus 6.Superior oblique 7.Inferior oblique LEVATOR PALPEBRAE SUPERIORIOS Origin- Inferior surface of lesser wing of sphenoid. Insertion- Upper lamina (Voluntary) - Anterior surface of superior tarsus & skin of upper eyelid. Middle lamina (Involuntary) - Superior margin of superior tarsus. (Superior Tarsus Muscle / Muller muscle) Lower lamina (Involuntary) - Superior conjunctival fornix Nerve Supply :- Voluntary part – Oculomotor Nerve Involuntary part – Sympathetic ACTION :- Elevation of upper eye lid C/S :- Drooping of upper eyelid. Congenital ptosis due to localized myogenic dysgenesis Complete ptosis - Injury to occulomotor nerve. Partial ptosis - disruption of postganglionic sympathetic fibres from superior cervical sympathetic ganglion. Extra ocular Muscles : Origin Levator palpebrae superioris Superior Oblique Superior Rectus Lateral Rectus Medial Rectus Inferior Oblique Inferior Rectus RECTUS MUSCLES : ORIGIN • Arises from a common tendinous ring knows as ANNULUS OF ZINN • Common ring of connective tissue • Anterior to optic foramen • Forms a muscle cone Clinical Significance Retrobulbar neuritis ○ Origin of SUPERIOR AND MEDIAL RECTUS are closely attached to the dural sheath of the optic nerve, which leads to pain during upward & inward movements of the globe. Thyroid orbitopathy ○ Medial & Inf.rectus thicken. especially near the orbital apex - compression of the optic nerve as it enters the optic canal adjacent to the body of the sphenoid bone. Ophthalmoplegia ○ Proptosis occur due to muscle laxity. Medial Rectus Superior Rectus Origin :- Superior limb of the tendonous ring, and optic nerve sheath. -
Periorbital Sinuses the Periorbital Sinuses Have a Close Anatomical Relationship with the Orbits (Fig 1-8)
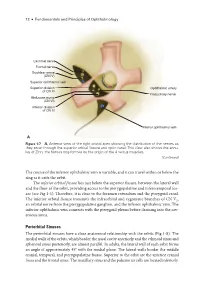
12 ● Fundamentals and Principles of Ophthalmology Lacrimal nerve Frontal nerve Trochlear nerve (CN IV) Superior ophthalmic vein Superior division Ophthalmic artery of CN III Nasociliary nerve Abducens nerve (CN VI) Inferior division of CN III Inferior ophthalmic vein A Figure 1-7 A, Anterior view of the right orbital apex showing the distribution of the nerves as they enter through the superior orbital fissure and optic canal. This view also shows the annu- lus of Zinn, the fibrous ring formed by the origin of the 4 rectus muscles. (Continued) The course of the inferior ophthalmic vein is variable, and it can travel within or below the ring as it exits the orbit. The inferior orbital fissure lies just below the superior fissure, between the lateral wall and the floor of the orbit, providing access to the pterygopalatine and inferotemporal fos- sae (see Fig 1-1). Therefore, it is close to the foramen rotundum and the pterygoid canal. The inferior orbital fissure transmits the infraorbital and zygomatic branches of CN V2, an orbital nerve from the pterygopalatine ganglion, and the inferior ophthalmic vein. The inferior ophthalmic vein connects with the pterygoid plexus before draining into the cav- ernous sinus. Periorbital Sinuses The periorbital sinuses have a close anatomical relationship with the orbits (Fig 1-8). The medial walls of the orbits, which border the nasal cavity anteriorly and the ethmoid sinus and sphenoid sinus posteriorly, are almost parallel. In adults, the lateral wall of each orbit forms an angle of approximately 45° with the medial plane. The lateral walls border the middle cranial, temporal, and pterygopalatine fossae. -

Eye-Movement Research: an Overview of Current and Past Developments
Elsevier AMS Ch01-I044980 Job code: EMAW 14-2-2007 1:04p.m. Page:1 Trimsize:165×240MM Chapter 1 EYE-MOVEMENT RESEARCH: AN OVERVIEW OF CURRENT AND PAST DEVELOPMENTS ROGER P. G. VAN GOMPEL, MARTIN H. FISCHER AND WAYNE S. MURRAY University of Dundee, UK ROBIN L. HILL University of Edinburgh, UK Eye Movements: A Window on Mind and Brain Edited by R. P. G. van Gompel, M. H. Fischer, W. S. Murray and R. L. Hill Copyright © 2007 by Elsevier Ltd. All rights reserved. Basal Fonts:Times Margins:Top:4.6pc Gutter:4.6pc Font Size:10/12 Text Width:30pc Depth:43 Lines Elsevier AMS Ch01-I044980 Job code: EMAW 14-2-2007 1:04p.m. Page:2 Trimsize:165×240MM 2 R. P. G. van Gompel et al. Abstract This opening chapter begins with an overview of the issues and questions addressed in the remainder of this book, the contents of which reflect the wide diversity of eye-movement research. We then provide the reader with an up-to-date impression of the most significant developments in the area, based on findings from a survey of eye-movement researchers and database searches. We find that the most heavily cited publications are not necessarily those rated as most influential. It is clear that eye-movement research is published across a wide variety of journals and the number of articles is increasing, but the relative proportion of eye-movement articles has remained almost constant. The United States produces the largest number of these articles, but other countries actually produce more per capita. -

The Fovea in Retinopathy of Prematurity
Multidisciplinary Ophthalmic Imaging The Fovea in Retinopathy of Prematurity James D. Akula,1,2 Ivana A. Arellano,1 Emily A. Swanson,1 Tara L. Favazza,1 Theodore S. Bowe,2 Robert J. Munro,1 R. Daniel Ferguson,3 Ronald M. Hansen,1,2 Anne Moskowitz,1,2 and Anne B. Fulton1,2 1Department of Ophthalmology, Boston Children’s Hospital, Boston, Massachusetts, United States 2Department of Ophthalmology, Harvard Medical School, Boston, Massachusetts, United States 3Department of Biomedical Optics, Physical Sciences, Inc., Andover, Massachusetts, United States Correspondence: James D. Akula, PURPOSE. Because preterm birth and retinopathy of prematurity (ROP) are associated Department of Ophthalmology, with poor visual acuity (VA) and altered foveal development, we evaluated relationships Boston Children’s Hospital, among the central retinal photoreceptors, postreceptor retinal neurons, overlying fovea, 300 Longwood Aveue, Fegan 4, andVAinROP. Boston, MA 02115, USA; [email protected]. METHODS. We obtained optical coherence tomograms (OCTs) in preterm born subjects with no history of ROP (none; n = 61), ROP that resolved spontaneously without treat- Received: December 18, 2019 ment (mild; n = 51), and ROP that required treatment by laser ablation of the avascu- Accepted: July 2, 2020 = Published: September 16, 2020 lar peripheral retina (severe; n 22), as well as in term born control subjects (term; n = 111). We obtained foveal shape descriptors, measured central retinal layer thick- Citation: Akula JD, Arellano IA, nesses, and demarcated the anatomic parafovea using automated routines. In subsets Swanson EA, et al. The fovea in = retinopathy of prematurity. Invest of these subjects, we obtained OCTs eccentrically through the pupil (n 46) to reveal Ophthalmol Vis Sci. -

Clinical Study Photoreceptor Inner and Outer Segment Junction Reflectivity After Vitrectomy for Macula-Off Rhegmatogenous Retinal Detachment
Hindawi Publishing Corporation Journal of Ophthalmology Volume 2015, Article ID 451408, 7 pages http://dx.doi.org/10.1155/2015/451408 Clinical Study Photoreceptor Inner and Outer Segment Junction Reflectivity after Vitrectomy for Macula-Off Rhegmatogenous Retinal Detachment Jakub J. Kaluzny,1,2 Bartosz L. Sikorski,3 Grzegorz Czajkowski,2 Mateusz Burduk,3 Bartlomiej J. Kaluzny,3 Joanna Stafiej,3 and Grazyna Malukiewicz3 1 Department of Public Health, Collegium Medicum, Nicolaus Copernicus University, Ulica Sandomierska 16, 85-830 Bydgoszcz, Poland 2Oftalmika Eye Hospital, Ulica Modrzewiowa 15, 85-631 Bydgoszcz, Poland 3Department of Ophthalmology, Collegium Medicum, Nicolaus Copernicus University, Ulica Marii Curie-Skłodowskiej 9, 85-094 Bydgoszcz, Poland Correspondence should be addressed to Jakub J. Kaluzny; [email protected] and Bartosz L. Sikorski; [email protected] Received 4 May 2015; Revised 26 June 2015; Accepted 1 July 2015 AcademicEditor:LawrenceS.Morse Copyright © 2015 Jakub J. Kaluzny et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Purpose. To evaluate the spatial distribution of photoreceptor inner and outer segment junction (IS/OS) reflectivity changes after successful vitrectomy for macula-off retinal detachment (PPV-mOFF) using spectral domain optical coherence tomography (SdOCT). Methods. Twenty eyes after successful PPV-mOFF were included in the study. During a mean follow-up period of 15.3 months, SdOCT was performed four times. To evaluate the IS/OS reflectivity a four-grade scale was used. Results. At the first follow- up visit the IS/OS had very similar reflectivity in entire length of the central scan with total average value of 1,05. -

98796-Anatomy of the Orbit
Anatomy of the orbit Prof. Pia C Sundgren MD, PhD Department of Diagnostic Radiology, Clinical Sciences, Lund University, Sweden Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lay-out • brief overview of the basic anatomy of the orbit and its structures • the orbit is a complicated structure due to its embryological composition • high number of entities, and diseases due to its composition of ectoderm, surface ectoderm and mesoderm Recommend you to read for more details Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 3 x 3 Imaging technique 3 layers: - neuroectoderm (retina, iris, optic nerve) - surface ectoderm (lens) • CT and / or MR - mesoderm (vascular structures, sclera, choroid) •IOM plane 3 spaces: - pre-septal •thin slices extraconal - post-septal • axial and coronal projections intraconal • CT: soft tissue and bone windows 3 motor nerves: - occulomotor (III) • MR: T1 pre and post, T2, STIR, fat suppression, DWI (?) - trochlear (IV) - abducens (VI) Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Superior orbital fissure • cranial nerves (CN) III, IV, and VI • lacrimal nerve • frontal nerve • nasociliary nerve • orbital branch of middle meningeal artery • recurrent branch of lacrimal artery • superior orbital vein • superior ophthalmic vein Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. -

Embryology, Anatomy, and Physiology of the Afferent Visual Pathway
CHAPTER 1 Embryology, Anatomy, and Physiology of the Afferent Visual Pathway Joseph F. Rizzo III RETINA Physiology Embryology of the Eye and Retina Blood Supply Basic Anatomy and Physiology POSTGENICULATE VISUAL SENSORY PATHWAYS Overview of Retinal Outflow: Parallel Pathways Embryology OPTIC NERVE Anatomy of the Optic Radiations Embryology Blood Supply General Anatomy CORTICAL VISUAL AREAS Optic Nerve Blood Supply Cortical Area V1 Optic Nerve Sheaths Cortical Area V2 Optic Nerve Axons Cortical Areas V3 and V3A OPTIC CHIASM Dorsal and Ventral Visual Streams Embryology Cortical Area V5 Gross Anatomy of the Chiasm and Perichiasmal Region Cortical Area V4 Organization of Nerve Fibers within the Optic Chiasm Area TE Blood Supply Cortical Area V6 OPTIC TRACT OTHER CEREBRAL AREASCONTRIBUTING TO VISUAL LATERAL GENICULATE NUCLEUSPERCEPTION Anatomic and Functional Organization The brain devotes more cells and connections to vision lular, magnocellular, and koniocellular pathways—each of than any other sense or motor function. This chapter presents which contributes to visual processing at the primary visual an overview of the development, anatomy, and physiology cortex. Beyond the primary visual cortex, two streams of of this extremely complex but fascinating system. Of neces- information flow develop: the dorsal stream, primarily for sity, the subject matter is greatly abridged, although special detection of where objects are and for motion perception, attention is given to principles that relate to clinical neuro- and the ventral stream, primarily for detection of what ophthalmology. objects are (including their color, depth, and form). At Light initiates a cascade of cellular responses in the retina every level of the visual system, however, information that begins as a slow, graded response of the photoreceptors among these ‘‘parallel’’ pathways is shared by intercellular, and transforms into a volley of coordinated action potentials thalamic-cortical, and intercortical connections. -

A Model of Discourse Predictions in Human Sentence Processing
A Model of Discourse Predictions in Human Sentence Processing Amit Dubey and Frank Keller and Patrick Sturt Human Communication Research Centre, University of Edinburgh 10 Crichton Street, Edinburgh EH8 9AB, UK {amit.dubey,frank.keller,patrick.sturt}@ed.ac.uk Abstract prediction has been demonstrated by studies that show anticipation based on selectional restrictions: This paper introduces a psycholinguistic listeners are able to launch eye-movements to the model of sentence processing which combines predicted argument of a verb before having encoun- a Hidden Markov Model noun phrase chun- tered it, e.g., they will fixate an edible object as soon ker with a co-reference classifier. Both mod- els are fully incremental and generative, giv- as they hear the word eat (Altmann and Kamide, ing probabilities of lexical elements condi- 1999). Semantic prediction has also been shown in tional upon linguistic structure. This allows the context of semantic priming: a word that is pre- us to compute the information theoretic mea- ceded by a semantically related prime or by a seman- sure of surprisal, which is known to correlate tically congruous sentence fragment is processed with human processing effort. We evaluate faster (Stanovich and West, 1981; Clifton et al., our surprisal predictions on the Dundee corpus 2007). An example for syntactic prediction can be of eye-movement data show that our model achieve a better fit with human reading times found in coordinate structures: readers predict that than a syntax-only model which does not have the second conjunct in a coordination will have the access to co-reference information. -

Vision Research 138 (2017) 59–65
Vision Research 138 (2017) 59–65 Contents lists available at ScienceDirect Vision Research journal homepage: www.elsevier.com/locate/visres Role of parafovea in blur perception ⇑ Abinaya Priya Venkataraman a, ,1, Aiswaryah Radhakrishnan b,1, Carlos Dorronsoro b, Linda Lundström a, Susana Marcos b a Department of Applied Physics, KTH, Royal Institute of Technology, Stockholm, Sweden b Visual Optics and Biophotonics Lab, Instituto de Óptica ‘‘Daza de Valdés”, Consejo Superior de Investigaciones Científicas, Madrid, Spain article info abstract Article history: The blur experienced by our visual system is not uniform across the visual field. Additionally, lens designs Received 22 April 2017 with variable power profile such as contact lenses used in presbyopia correction and to control myopia Received in revised form 10 July 2017 progression create variable blur from the fovea to the periphery. The perceptual changes associated with Accepted 15 July 2017 varying blur profile across the visual field are unclear. We therefore measured the perceived neutral focus with images of different angular subtense (from 4° to 20°) and found that the amount of blur, for which Number of reviewers = 2 focus is perceived as neutral, increases when the stimulus was extended to cover the parafovea. We also studied the changes in central perceived neutral focus after adaptation to images with similar magnitude Keywords: of optical blur across the image or varying blur from center to the periphery. Altering the blur in the Blur adaptation Peripheral blur periphery had little or no effect on the shift of perceived neutral focus following adaptation to normal/ Central vision blurred central images. -

Research Article Increased Subfoveal Choroidal Thickness and Retinal Structure Changes on Optical Coherence Tomography in Pediatric Alport Syndrome Patients
Hindawi Journal of Ophthalmology Volume 2019, Article ID 6741930, 7 pages https://doi.org/10.1155/2019/6741930 Research Article Increased Subfoveal Choroidal Thickness and Retinal Structure Changes on Optical Coherence Tomography in Pediatric Alport Syndrome Patients Seda Karaca Adıyeke ,1 Gamze Ture,1 Fatma Mutlubas¸,2 Hasan Aytog˘an,1 Onur Vural,1 Neslisah Kutlu Uzakgider,1 Gulsah Talay Dayangaç,1 and Ekrem Talay1 1Tepecik Research and Training Hospital, Ophthalmology Department, Izmir, Turkey 2Tepecik Research and Training Hospital, Pediatric Nephrology Department, Izmir, Turkey Correspondence should be addressed to Seda Karaca Adıyeke; [email protected] Received 8 September 2018; Revised 19 November 2018; Accepted 17 December 2018; Published 21 January 2019 Academic Editor: Sentaro Kusuhara Copyright © 2019 Seda Karaca Adıyeke et al. )is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Objective. To evaluate optical coherence tomography (OCT) findings of pediatric Alport syndrome (AS) patients with no retinal pathology on fundus examination. Materials and Methods. Twenty-one patients being followed up with the diagnosis of AS (Group 1) and 24 age- and sex-matched healthy volunteers (Group 2) were prospectively evaluated. All participants underwent standard ophthalmologic examination, retinal nerve fibre layer (RNFL) analysis, and horizontal and vertical scan macula en- hanced depth imaging OCT (EDI-OCT). Statistical analysis of the data obtained in this study was performed with SPSS 15.0. Results. Macula thickness was significantly decreased in the temporal quadrant in Group 1 compared to those of the control group (p � 0:013). -

(AMD) and a New Metric for Objective Evaluation of the Efficacy of Ocular Nutrition
Nutrients 2012, 4, 1812-1827; doi:10.3390/nu4121812 OPEN ACCESS nutrients ISSN 2072-6643 www.mdpi.com/journal/nutrients Article Retinal Spectral Domain Optical Coherence Tomography in Early Atrophic Age-Related Macular Degeneration (AMD) and a New Metric for Objective Evaluation of the Efficacy of Ocular Nutrition Stuart Richer 1,2,*, Jane Cho 1,2, William Stiles 1, Marc Levin 1, James S. Wrobel 3, Michael Sinai 4 and Carla Thomas 1 1 Eye Clinic, James A Lovell Federal Health Care Center, North Chicago, IL 60064, USA; E-Mails: [email protected] (J.C.); [email protected] (W.S.); [email protected] (M.L.); [email protected] (C.T.) 2 Family & Preventive Medicine, RFUMS Chicago Medical School, North Chicago, IL 60064, USA 3 Internal Medicine, Podiatry Services, University of Michigan, Ann Arbor, MI 48105, USA; E-Mail: [email protected] 4 Optovue Inc., Fremont, CA 94538, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-224-610-7145. Received: 11 October 2012; in revised form: 12 November 2012 / Accepted: 14 November 2012 / Published: 27 November 2012 Abstract: Purpose: A challenge in ocular preventive medicine is identification of patients with early pathological retinal damage that might benefit from nutritional intervention. The purpose of this study is to evaluate retinal thinning (RT) in early atrophic age-related macular degeneration (AMD) against visual function data from the Zeaxanthin and Visual Function (ZVF) randomized double masked placebo controlled clinical trial (FDA IND #78973). Methods: Retrospective, observational case series of medical center veterans with minimal visible AMD retinopathy (AREDS Report #18 simplified grading 1.4/4.0 bilateral retinopathy).