Valeric Acid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 21 the Chemistry of Carboxylic Acid Derivatives
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 21 The Chemistry of Carboxylic Acid Derivatives Solutions to In-Text Problems 21.1 (b) (d) (e) (h) 21.2 (a) butanenitrile (common: butyronitrile) (c) isopentyl 3-methylbutanoate (common: isoamyl isovalerate) The isoamyl group is the same as an isopentyl or 3-methylbutyl group: (d) N,N-dimethylbenzamide 21.3 The E and Z conformations of N-acetylproline: 21.5 As shown by the data above the problem, a carboxylic acid has a higher boiling point than an ester because it can both donate and accept hydrogen bonds within its liquid state; hydrogen bonding does not occur in the ester. Consequently, pentanoic acid (valeric acid) has a higher boiling point than methyl butanoate. Here are the actual data: INSTRUCTOR SUPPLEMENTAL SOLUTIONS TO PROBLEMS • CHAPTER 21 2 21.7 (a) The carbonyl absorption of the ester occurs at higher frequency, and only the carboxylic acid has the characteristic strong, broad O—H stretching absorption in 2400–3600 cm–1 region. (d) In N-methylpropanamide, the N-methyl group is a doublet at about d 3. N-Ethylacetamide has no doublet resonances. In N-methylpropanamide, the a-protons are a quartet near d 2.5. In N-ethylacetamide, the a- protons are a singlet at d 2. The NMR spectrum of N-methylpropanamide has no singlets. 21.9 (a) The first ester is more basic because its conjugate acid is stabilized not only by resonance interaction with the ester oxygen, but also by resonance interaction with the double bond; that is, the conjugate acid of the first ester has one more important resonance structure than the conjugate acid of the second. -

Valproic Acid and Its Amidic Derivatives As New Antivirals Against Alphaherpesviruses
viruses Review Valproic Acid and Its Amidic Derivatives as New Antivirals against Alphaherpesviruses Sabina Andreu 1,2,* , Inés Ripa 1,2, Raquel Bello-Morales 1,2 and José Antonio López-Guerrero 1,2 1 Departamento de Biología Molecular, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain; [email protected] (I.R.); [email protected] (R.B.-M.); [email protected] (J.A.L.-G.) 2 Centro de Biología Molecular Severo Ochoa, Spanish National Research Council—Universidad Autónoma de Madrid (CSIC-UAM), Cantoblanco, 28049 Madrid, Spain * Correspondence: [email protected] Academic Editor: Maria Kalamvoki Received: 14 November 2020; Accepted: 25 November 2020; Published: 26 November 2020 Abstract: Herpes simplex viruses (HSVs) are neurotropic viruses with broad host range whose infections cause considerable health problems in both animals and humans. In fact, 67% of the global population under the age of 50 are infected with HSV-1 and 13% have clinically recurrent HSV-2 infections. The most prescribed antiherpetics are nucleoside analogues such as acyclovir, but the emergence of mutants resistant to these drugs and the lack of available vaccines against human HSVs has led to an imminent need for new antivirals. Valproic acid (VPA) is a branched short-chain fatty acid clinically used as a broad-spectrum antiepileptic drug in the treatment of neurological disorders, which has shown promising antiviral activity against some herpesviruses. Moreover, its amidic derivatives valpromide and valnoctamide also share this antiherpetic activity. This review summarizes the current research on the use of VPA and its amidic derivatives as alternatives to traditional antiherpetics in the fight against HSV infections. -

The Lysis of Bacterium Coli by Amino-Acids
[510 ] THE LYSIS OF BACTERIUM COLI BY AMINO-ACIDS BY J. GORDON, R. A. HALL AND L. H. STICKLAND The School of Medicine, Leeds (With 2 Figures in the Text) Gordon, Hall & Stickland (1951, 1953) described the kinetics of the lysis by glycine of Bacterium coli suspensions, and the inhibition by various agents of this process. The present paper attempts to correlate this lytic effect with the chemical structure of various amino-acids. MATERIALS AND METHODS Preparation of bacteria Four freshly isolated strains of Bact. coli were grown on the surface of nutrient agar for 16-18 hr. at 37°, and were then washed off with distilled water. These suspensions were filtered through glass wool. The bacteria were washed twice and finally suspended in about ten times their volume of distilled water which was equivalent to a concentration of between 10 and 20 mg. dry weight of cells per ml. Measurement of lysis After the suspensions had been incubated with the amino-acid solutions under test the bacteria were removed by centrifuging at 2500 g. for 20 min. The super- natant fluid was treated with \ volume of 25 % ' w/v' trichloracetic acid solution, the protein precipitate was washed with 5% trichloracetic acid and then the protein was estimated by the biuret method (Robinson & Hogden, 1940). The degree of lysis was expressed as the ratio of the protein liberated into the super- natant fluid to the total soluble protein of the bacterial suspension (Stickland, 1951). Amino-acids used Glycine, DL-alanine, /?-alanine, DL-a-amino-w-butyric acid, DL-a-amino-iso- butyric acid, DL-a-amino-?i-valeric acid, DL-valine, DL-serine, glycine ethyl ester hydrochloride, aceturic acid, glycylglycine and L-glutamic acid were obtained commercially. -

A Facile Profiling Method of Short Chain Fatty Acids Using Liquid
H OH metabolites OH Article A Facile Profiling Method of Short Chain Fatty Acids Using Liquid Chromatography-Mass Spectrometry Ha Eun Song 1, Hyo Yeong Lee 1, Su Jung Kim 1, Sung Hoon Back 2 and Hyun Ju Yoo 1,* 1 Department of Convergence Medicine, Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea 2 School of Biological Sciences, University of Ulsan, Ulsan 44610, Korea * Correspondence: [email protected]; Tel.: +82-02-3010-4029 Received: 27 June 2019; Accepted: 23 August 2019; Published: 28 August 2019 Abstract: Short chain fatty acids (SCFAs) are the main products of dietary fibers that are not digested by the human body, and they have been shown to affect human metabolism and inflammation. The amount of SCFAs in the body is related to many human diseases, and studies have focused on elucidating their roles and target molecules in both metabolic and immune responses. Thus, the quantitation of SCFAs in biological samples becomes crucial in understanding their important roles in the human body. Herein, a facile profiling method of SCFAs using liquid chromatography-tandem mass spectrometry (LC-MS/MS) was developed and then applied to biological samples. C2-C6 SCFAs were derivatized while using 4-acetamido-7-mercapto-2,1,3-benzoxadiazole for 5 min. at room temperature prior to LC-MS/MS analysis, and characteristic fragmentation patterns and increased hydrophobicity after chemical derivatization enabled specific discrimination among 12 SCFAs. Derivatization was fast and reliable, and the reaction products were stable for a week at 4 ◦C. -

Valerolactone Hydrogenation Into 1,4-Pentanediol in Milder Reaction Conditions
reactions Article Heterogeneously Catalyzed γ-Valerolactone Hydrogenation into 1,4-Pentanediol in Milder Reaction Conditions Irina Simakova 1,2,* , Yulia Demidova 1,2, Mikhail Simonov 1,2 , Sergey Prikhod’ko 1,2, Prashant Niphadkar 3, Vijay Bokade 3, Paresh Dhepe 3 and Dmitry Yu. Murzin 4,* 1 Boreskov Institute of Catalysis, pr. Lavrentieva, 5, 630090 Novosibirsk, Russia; [email protected] (Y.D.); [email protected] (M.S.); [email protected] (S.P.) 2 Novosibirsk State University, Pirogova 2, 630090 Novosibirsk, Russia 3 CSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411008, India; [email protected] (P.N.); [email protected] (V.B.); [email protected] (P.D.) 4 Johan Gadolin Process Chemistry Centre, Åbo Akademi University, Biskopsgatan 8, FI-20500 Turku/Åbo, Finland * Correspondence: [email protected] (I.S.); dmurzin@abo.fi (D.Y.M.) Received: 20 September 2020; Accepted: 14 October 2020; Published: 16 October 2020 Abstract: Hydrogenation of γ-valerolactone (GVL) in polar solvents (n-butanol, 1,4-dioxane) to 1,4-pentanediol (PDO) and 2-methyltetrahydrofuran (MTHF) was performed at 363–443 K in a fixed bed reactor under overall H2 pressure of 0.7–1.3 MPa. Preliminary screening in a batch reactior was performed with a series of Ru, Ir, Pt, Co, and Cu catalysts, earlier efficiently applied for levulinic acid hydrogenation to GVL. The fresh catalysts were analyzed by transmission electron microscopy (TEM), X-ray fluorescent analysis (XRF), temperature programmed reduction by H2 (H2-TPR), and N2 physisorption. Cu/SiO2 prepared by reduction of copper hydroxosilicate with chrysocolla mineral structure provided better selectivity of 67% towards PDO at 32% GVL conversion in a continuous flow reactor. -
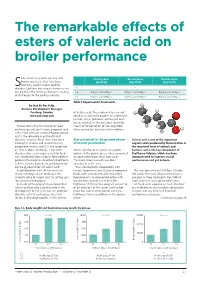
The Remarkable Effects of Esters of Valeric Acid on Broiler Performance
The remarkable effects of esters of valeric acid on broiler performance hort chain fatty acids are very well- Starter phase Grower phase Finisher phase known molecules that have been (day 0-14) (day 15-28) (day 29-42) routinely used in animal feed for S T-1 - - - decades. Each one has unique characteristics making them the reference to tackle an array T-2 Butyric acid 500g/t Butyric acid 500g/t Butyric acid 250g/t of challenges to the poultry industry. T-3 Butyric acid 500g/t Valeric acid 1500g/t Butyric acid 250g/t Table 1. Experimental treatments. by José M. Ros Felip, Business Development Manager, Perstorp, Sweden. of butyric acid. These observations do not www.perstorp.com come as a surprise to experts, as valeric acid, just like acetic, propionic and butyric acid, occurs naturally in the intestinal lumen by Formic acid is the first choice for feed means of fermentation of non-digestible acidification and sanitisation, propionic acid fibres carried out by intestinal microbiota. is the most efficient mould-inhibitor, butyric acid is the reference in gut health and digestive integrity. All of them have been Star potential in the grower phase Valeric acid is one of the important thoroughly studied and used intensively of broiler production organic acids produced by fermentation in except one, valeric acid (C5), the longest of the intestinal tract of animals and all. That is about to change. It has been Valeric acid has been shown to support humans, and is the key component of decades since a new organic acid for feed broilers in the grower phase when compared ProPhorce Valerins, which has been was introduced. -

Handheld Odor Monitor FAQ Frequently Asked Questions
1 High Temperature Application Guide Handheld Odor Monitor FAQ Frequently Asked Questions 1. What is the correlation between the odor strength value from OMX-SRM and value of odor intensity, odor concentration or other odor index? There is no correlation between the indicated value of OMX- SRM and value of human sense of odor intensity, odor concentration or other odor index. OMX-SRM indicates relative value by comparing odor gas to purified air. This measurement is based on our original methodology. “Smell” is the information by human sense (sensitivity value), and there is no method that can accurately quantify various kinds of smells. There are some expressions such as “odor intensity” and “odor concentration” which are quantified based on human sense of smell. The OMX- SRM focuses on chemical substances contained in a smell and this device shows the relative strength of a smell numerically by using semiconductor gas sensors. The reading shown on display is calculated from the output from gas sensors using our original measurement to generate a numerical coefficient. 2. When OMX-SRM displays value of 300 for strength, what would be “odor intensity” or “odor concentration” in general? Also what unit is used for this figures, ppm? It is impossible to answer the first question from the perspective of the various points when measuring a compound smell. Even if device displays 300, you may perceive it as “an intense smell” or as “a week smell” depending on the kind of smell. OMX Handheld Odor Monitor Smell has the following characteristics; 1.Smell is a gas consisting of multiple ingredients. -

Formic Acid Acetic Acid Propionic Acid Butyric Acid Valeric Acid Caproic
Organic Acids: Formic acid Citric acid Acetic acid Malic acid Propionic acid Benzoic acid Butyric acid Tartaric acid Valeric acid Caproic acid Oxalic acid Lactic acid Organic Bases: Pyridine Imidazole (solid) Benzimidazole Aniline TEA (tri-ethyl amine) Histidine Nitroaniline Imidazole (dissolved) Amino bases/ Nucleotides ממסים אורגניים (מכילים קשר פחמן-מימן): :(Organic Solvents (contains carbon-hydrogen bond Xylene DMF Hexane Parafine oil Ethylacetate Formamide IPA Piperidine Trizol/Trireagent Butanol SDS (solid) Acetone PMSF (Phenylmethanesulfonyl fluoride) Methanol Tween PFA RNA / DNA (kit parts that contains thioisocyanate) Toluene BME (beta mercaptoethanol) DMSO Ethylenglycol Ethanol Halogenated Organic Solvents (contains F, Cl, Br, I): ממסים אורגניים הלוגניים (מכילים F, Cl, Br, I) Chloroform (CHCl3) Methylene chloride Vinyl chloride Tetrafluoroethylene (CF2 =CF2) Trichloroethylene (CHCl=CCl2) Bromoethane Tert-Butyl bromide חומרים אנאורגניים (חומרים שהם לא חומצה/בסיס אנאורגני) Inorganic Materials (are not inorganic acid/base) LiCl Salts (such as MgCl2, CaCl) Hydrogen peroxide (H2O2) Metals (Cu, Pb, Na etc.) Ammonium thiocyanate (NH4SCN) Sodium Azide (NaN3) Ammonium azide (NH4N3) בסיסים אנאורגניים :Inorganic Bases NH3 NaOH NH4OH Ba(OH)2 NaOH (dissolved) Ca(OH)2 KOH (dissolved) CaCO3 KOH חומצות אנאורגניות: :Inorganic Acids Boric acid (H3BO3) Hydrochloric acid (HCl) Hydrofluoric acid (HF) Nitric acid (HNO3) Hydrobromic acid (HBr) Phosphoric acid (H3PO4) Perchloric acid (HClO4) Sulfuric acid (H2SO4) Hydroiodic acid (HI) Cytotoxic materials: -

Short-Chain Fatty Acids Modulate Metabolic Pathways and Membrane Lipids in Prevotella Bryantii B14
proteomes Article Short-Chain Fatty Acids Modulate Metabolic Pathways and Membrane Lipids in Prevotella bryantii B14 Andrej Trautmann 1, Lena Schleicher 2, Simon Deusch 1 , Jochem Gätgens 3 , Julia Steuber 2 and Jana Seifert 1,* 1 Institute of Animal Science, University of Hohenheim, 70599 Stuttgart, Germany; [email protected] (A.T.); [email protected] (S.D.) 2 Institute of Biology, University of Hohenheim, 70599 Stuttgart, Germany; [email protected] (L.S.); [email protected] (J.S.) 3 Institute of Bio- and Geosciences, IBG-1: Biotechnology, Forschungszentrum Jülich, 52425 Jülich, Germany; [email protected] * Correspondence: [email protected] Received: 22 September 2020; Accepted: 13 October 2020; Published: 16 October 2020 Abstract: Short-chain fatty acids (SCFAs) are bacterial products that are known to be used as energy sources in eukaryotic hosts, whereas their role in the metabolism of intestinal microbes is rarely explored. In the present study, acetic, propionic, butyric, isobutyric, valeric, and isovaleric acid, respectively, were added to a newly defined medium containing Prevotella bryantii B14 cells. After 8 h and 24 h, optical density, pH and SCFA concentrations were measured. Long-chain fatty acid (LCFA) profiles of the bacterial cells were analyzed via gas chromatography-time of flight-mass spectrometry (GC-ToF MS) and proteins were quantified using a mass spectrometry-based, label-free approach. Cultures supplemented with single SCFAs revealed different growth behavior. Structural features of the respective SCFAs were identified in the LCFA profiles, which suggests incorporation into the bacterial membranes. The proteomes of cultures supplemented with acetic and valeric acid differed by an increased abundance of outer membrane proteins. -

2013 Pguy Acd Labs Presentation
‘Next Generation Fingerprinting’ A GC‐HR‐TOF‐MS Method to Semi‐Quantify Constituents in Aerosols and Aerosol Fractions E. Dossin, A. Monge, E. Martin, P. Pospisil, A. Knorr, M.C. Bentley, P.A. Guy Metabolomics & Analytical Chemistry Gr., Philip Morris Products SA, 2000 Neuchatel, Switzerland Outline 1. Description of the existing GC-MS fingerprinting method Pros & cons of the current fingerprinting method Why switching to a 7200 Agilent high resolution MS instrument? 2. How to tackle semi-quantification of the smoke constituents with the help of chemoinformatics tools Curation step for smoke constituents to be monitored Analysis of reference standards Retention time prediction model (QSPR approach) Selection of appropriate internal standards (clustering approach) 3. Semi-quantification from calibration curve of reference standards Linearity Assessment of silylation 4. What about other smoke constituents? 5. Conclusion Page: 2 Existing Fingerprinting Method • Aerosol sample generated from a smoking machine (ISO) – Whole smoke – Gas Vapor Phase (GVP) sbPBS – Total Particulate Matter (TPM) • Compounds list • GC columns (HP6890 GC) – DB-624: HS-SPME-GC-MS Volatile Chemicals – DB-FFAP: GC-MS Non Polar Chemicals –DB-5-MS: HT-GC-MS Polar Chemicals (TMS) • Detection (MSD5973 MS) – Electron ionization mode – Full scan (low resolution) Semi-quantification (d6-phenol) Page: 3 Description of the Former GC‐MS Fingerprinting Method (HP6890 GC ‐ MSD5973 MS) Cell culture Medium plus Cigarette DB-624 column ● Headspace(HS)-SPME-GC-MS Smoke high -

Fecal Short Chain Fatty Acids in Children Living on Farms and a Link
www.nature.com/scientificreports OPEN Fecal short chain fatty acids in children living on farms and a link between valeric acid and protection from eczema Monica Gio‑Batta1*, Fei Sjöberg1, Karin Jonsson2, Malin Barman2,3, Anna‑Carin Lundell4, Ingegerd Adlerberth1, Bill Hesselmar5, Ann‑Sofe Sandberg2 & Agnes E. Wold1 Children growing up on farms have low rates of allergy, but the mechanism for this protective efect has not been fully elucidated. Short chain fatty acids (SCFAs) produced by the gut microbiota may play a role in protection from allergy. We measured fecal SCFA levels in samples collected from 28 farming and 37 control children over the frst 3 years of life using gas chromatography. Data on diet and other host factors were recorded and allergy was diagnosed at 8 years of age. Among all children, median propionic and butyric acid concentration increased over the frst 3 years, and longer SCFAs typically appeared by 1 year of age. Farm children had higher levels of iso‑butyric, iso‑valeric and valeric acid at 3 years of age than rural controls. In addition, children with elder siblings had higher levels of valeric acid at 3 years of age, and dietary factors also afected SCFA pattern. High levels of valeric acid at 3 years of age were associated with low rate of eczema at 8 years of age. The fecal SCFA pattern in farm children suggests a more rapid maturation of the gut microbiota. Valeric acid or associated microbes may have protective potential against eczema. Living on a farm is protective against immunoregulatory and infammatory diseases, including allergic diseases 1,2 and infammatory bowel disease (IBD)3. -

Isolation, Identification, and Studies on the Metabolism of Rumen Micro-Organism Growth Factors Present in Natural Materials
ISOLATION, IDENTIFICATION, AND STUDIES ON THE METABOLISM OF RUMEN MICRO-ORGANISM GROWTH FACTORS PRESENT IN NATURAL MATERIALS DISSERTATION Presented In Partial Fuirillraent of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By BURK ALLYN DEHOR ITT, A.B., M.S. The Ohio State University 1957 Approved by: Adviser Department of Agricultural Biochemistry ACKNOWLEDGMENTS I would like to express ray deepest appreciation to Dr. Alvin L. Moxon and Dr. Orville G. Bentley for their guidance and many helpful suggestions during the per formance of ray research work. Their interest and leader ship is gratefully acknowledged. The timely suggestions and interest of Dr. Ronald R. Johnson are greatly appreciated, in addition to his aid in the preparation of Figures 10, 11, and 12. Appreciation is also extended to the Ohio Agricultural Experiment Station for providing facilities and financial assistance during the course of this work. I am indebted to ray wife for her unceasing encour agement and cheerfulness throughout the performance of this work, and her aid in the preparation of this manu script. ii TABLE OP CONTENTS General Introduction Part I: Isolation and .identification of Compounds Prom Autolyzed Yeast, Alfalfa Ileal, and Casoin iiydrolysa.te wit h. Cellulolytic Factor Activity for Rumen Micro-organisms In Vitro Literature Review Experimental Procedures In Vitro Rumen ^Fermentation Technique Fractionation Procedures Dowex - 50 I o n Exchange Resin Charcoal Treatment Preparative Scale