Atmospheric Traces Monitoring Using Cavity Ringdown Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beam Profiling by Michael Scaggs
Beam Profiling by Michael Scaggs Haas Laser Technologies, Inc. Introduction Lasers are ubiquitous in industry today. Carbon Dioxide, Nd:YAG, Excimer and Fiber lasers are used in many industries and a myriad of applications. Each laser type has its own unique properties that make them more suitable than others. Nevertheless, at the end of the day, it comes down to what does the focused or imaged laser beam look like at the work piece? This is where beam profiling comes into play and is an important part of quality control to ensure that the laser is doing what it intended to. In a vast majority of cases the laser beam is simply focused to a small spot with a simple focusing lens to cut, scribe, etch, mark, anneal, or drill a material. If there is a problem with the beam delivery optics, the laser or the alignment of the system, this problem will show up quite markedly in the beam profile at the work piece. What is Beam Profiling? Beam profiling is a means to quantify the intensity profile of a laser beam at a particular point in space. In material processing, the "point in space" is at the work piece being treated or machined. Beam profiling is accomplished with a device referred to a beam profiler. A beam profiler can be based upon a CCD or CMOS camera, a scanning slit, pin hole or a knife edge. In all systems, the intensity profile of the beam is analyzed within a fixed or range of spatial Haas Laser Technologies, Inc. -

Self Amplified Lock of a Ultra-Narrow Linewidth Optical Cavity
Self Amplified Lock of a Ultra-narrow Linewidth Optical Cavity Kiwamu Izumi,1, ∗ Daniel Sigg,1 and Lisa Barsotti2 1LIGO Hanford Observatory, PO Box 159 Richland, Washington 99354, USA 2LIGO laboratory, Massachusetts Institute of Technology, Cambridge, Massachussetts 02139, USA compiled: January 8, 2016 High finesse optical cavities are an essential tool in modern precision laser interferometry. The incident laser field is often controlled and stabilized with an active feedback system such that the field resonates in the cavity. The Pound-Drever-Hall reflection locking technique is a convenient way to derive a suitable error signal. However, it only gives a strong signal within the cavity linewidth. This poses a problem for locking a ultra-narrow linewidth cavity. We present a novel technique for acquiring lock by utilizing an additional weak control signal, but with a much larger capture range. We numerically show that this technique can be applied to the laser frequency stabilization system used in the Laser Interferometric Gravitational-wave Observatory (LIGO) which has a linewidth of 0.8 Hz. This new technique will allow us to robustly and repeatedly lock the LIGO laser frequency to the common mode of the interferometer. OCIS codes: (140.3425), (140.3410) http://dx.doi.org/10.1364/XX.99.099999 High finesse optical cavities have been an indispens- nonlinear response [8, 9] dominant and thus hinder the able tool for precision interferometry to conduct rela- linear controller. tivistic experiments such as gravitational wave detection Gravitational wave observatories deploy kilometer [1{3] and optical clocks [4]. The use of a high finesse cav- scale interferometers with extremely narrow linewidth. -

Absorption Spectroscopy and Imaging from the Visible Through Mid-Infrared with 20 Nm Resolution
Absorption spectroscopy and imaging from the visible through mid-infrared with 20 nm resolution. Aaron M. Katzenmeyer,1 Glenn Holland,1 Kevin Kjoller2 and Andrea Centrone1* 1Center for Nanoscale Science and Technology, National Institute of Standards and Technology, 100 Bureau Drive, Gaithersburg, Maryland 20899, United States. *E-mail: [email protected] 2Anasys Instruments, Inc., 325 Chapala Street, Santa Barbara, California 93101, United States. Abstract Absorption spectroscopy and mapping from visible through mid-IR wavelengths has been achieved with spatial resolution exceeding the limit imposed by diffraction, via the photothermal induced resonance technique. Correlated vibrational (chemical), and electronic properties are obtained simultaneously with topography with a wavelength-independent resolution of ≈ 20 nm using a single laboratory-scale instrument. This marks the highest resolution reported for PTIR, as determined by comparing height and PTIR images, and its first extension to near-IR and visible wavelengths. Light-matter interaction enables scientists to characterize materials and biological samples across a large range of energies. Visible (VIS) and near-infrared (NIR) light (from 400 nm to 2.5 µm) probes electronic transitions in materials [1], providing information regarding band gap, defects, and energy transfer which is crucial for the semiconductor and optoelectronic industries and for understanding processes such as photosynthesis. Mid-infrared (mid-IR) light (from 2.5 µm to 15 µm) probes vibrational transitions and provides rich chemical and structural information enabling materials identification [2]. Spectral maps can be obtained by coupling a light source and a spectrometer with an optical microscope [3]. However, light diffraction limits the lateral resolution achievable with conventional microscopic techniques to approximately half the wavelength of light [4]. -

Power Build-Up Cavity Coupled to a Laser Diode
I POWER BUILD-UP CAVITY COUPLED I TO A LASER DIODE Daniel J. Evans I Center of Excellence for Raman Technology University of Utah I Abstract combination of these elements will emit photons at different frequencies. The ends of these semiconductor In many Raman applications there is a need to devices are cleaved to form mirrors that bounce the I detect gases in the low ppb range. The desired photons back and forth within the cavity. The photons sensitivity can be achieved by using a high power laser excite more electrons, which form more photons source in the range of tens of watts. A system (referred to as optical pumping). 2 A certain portion of I combining a build-up cavity to enhance the power and the photons emit through the front and back cleaved an external cavity laser diode setup to narrow the surfaces of the laser diode. The amount of photons that bandwidth can give the needed power to the Raman get through the cleaved surfaces can be adjusted by I spectroscopy system. coating the surface or installing other mirrors. Introduction to Laser Diodes The planar cleaved surfaces of the laser diode form a Fabry-Perot cavity with set resonance frequencies I An important characteristic of all lasers is the (vp).3 The typical laser diode has a spacing of 150 f.1I11 mode structure. The mode structure refers to both the with an index of refraction of 3.5, yielding a resonance lasing frequency and the spatial characteristics of the frequency of 285 GHz. The wavelength spacing (~A.) I laser. -

UFS Lecture 13: Passive Modelocking
UFS Lecture 13: Passive Modelocking 6 Passive Mode Locking 6.2 Fast Saturable Absorber Mode Locking (cont.) (6.3 Soliton Mode Locking) 6.4 Dispersion Managed Soliton Formation 7 Mode-Locking using Artificial Fast Sat. Absorbers 7.1 Kerr-Lens Mode-Locking 7.1.1 Review of Paraxial Optics and Laser Resonator Design 7.1.2 Two-Mirror Resonators 7.1.3 Four-Mirror Resonators 7.1.4 The Kerr Lensing Effects (7.2 Additive Pulse Mode-Locking) 1 6.2.2 Fast SA mode locking with GDD and SPM Steady-state solution is chirped sech-shaped pulse with 4 free parameters: Pulse amplitude: A0 or Energy: W 2 = 2 A0 t Pulse width: t Chirp parameter : b Carrier-Envelope phase shift : y 2 Pulse width Chirp parameter Net gain after and Before pulse CE-phase shift Fig. 6.6: Modelocking 3 6.4 Dispersion Managed Soliton Formation in Fiber Lasers ~100 fold energy Fig. 6.12: Stretched pulse or dispersion managed soliton mode locking 4 Fig. 6.13: (a) Kerr-lens mode-locked Ti:sapphire laser. (b) Correspondence with dispersion-managed fiber transmission. 5 Today’s BroadBand, Prismless Ti:sapphire Lasers 1mm BaF2 Laser crystal: f = 10o OC 2mm Ti:Al2O3 DCM 2 PUMP DCM 2 DCM 1 DCM 1 DCM 2 DCM 1 BaF2 - wedges DCM 6 Fig. 6.14: Dispersion managed soliton including saturable absorption and gain filtering 7 Fig. 6.15: Steady state profile if only dispersion and GDD is involved: Dispersion Managed Soliton 8 Fig. 6.16: Pulse shortening due to dispersion managed soliton formation 9 7. -

Quantum Illumination at the Microwave Wavelengths
Quantum Illumination at the Microwave Wavelengths 1 2 3 4 5 6, Shabir Barzanjeh, Saikat Guha, Christian Weedbrook, David Vitali, Jeffrey H. Shapiro, and Stefano Pirandola ∗ 1Institute for Quantum Information, RWTH Aachen University, 52056 Aachen, Germany 2Quantum Information Processing Group, Raytheon BBN Technologies, Cambridge, Massachusetts 02138, USA 3QKD Corp., 60 St. George St., Toronto, M5S 3G4, Canada 4School of Science and Technology, University of Camerino, Camerino, Macerata 62032, Italy 5Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA 6Department of Computer Science & York Centre for Quantum Technologies, University of York, York YO10 5GH, United Kingdom Quantum illumination is a quantum-optical sensing technique in which an entangled source is exploited to improve the detection of a low-reflectivity object that is immersed in a bright thermal background. Here we describe and analyze a system for applying this technique at microwave frequencies, a more appropriate spectral region for target detection than the optical, due to the naturally-occurring bright thermal background in the microwave regime. We use an electro-opto- mechanical converter to entangle microwave signal and optical idler fields, with the former being sent to probe the target region and the latter being retained at the source. The microwave radiation collected from the target region is then phase conjugated and upconverted into an optical field that is combined with the retained idler in a joint-detection -

Lab 10: Spatial Profile of a Laser Beam
Lab 10: Spatial Pro¯le of a Laser Beam 2 Background We will deal with a special class of Gaussian beams for which the transverse electric ¯eld can be written as, r2 1 Introduction ¡ 2 E(r) = Eoe w (1) Equation 1 is expressed in plane polar coordinates Refer to Appendix D for photos of the appara- where r is the radial distance from the center of the 2 2 2 tus beam (recall r = x +y ) and w is a parameter which is called the spot size of the Gaussian beam (it is also A laser beam can be characterized by measuring its referred to as the Gaussian beam radius). It is as- spatial intensity pro¯le at points perpendicular to sumed that the transverse direction is the x-y plane, its direction of propagation. The spatial intensity pro- perpendicular to the direction of propagation (z) of ¯le is the variation of intensity as a function of dis- the beam. tance from the center of the beam, in a plane per- pendicular to its direction of propagation. It is often Since we typically measure the intensity of a beam convenient to think of a light wave as being an in¯nite rather than the electric ¯eld, it is more useful to recast plane electromagnetic wave. Such a wave propagating equation 1. The intensity I of the beam is related to along (say) the z-axis will have its electric ¯eld uni- E by the following equation, formly distributed in the x-y plane. This implies that " cE2 I = o (2) the spatial intensity pro¯le of such a light source will 2 be uniform as well. -

Construction of a Flashlamp-Pumped Dye Laser and an Acousto-Optic
; UNITED STATES APARTMENT OF COMMERCE oUBLICATION NBS TECHNICAL NOTE 603 / v \ f ''ttis oi Construction of a Flashlamp-Pumped Dye Laser U.S. EPARTMENT OF COMMERCE and an Acousto-Optic Modulator National Bureau of for Mode-Locking Iandards — NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measure- ment system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scien- tific community, industry, and commerce. The Institute consists of a Center for Radia- tion Research, an Office of Measurement Services and the following divisions: Applied Mathematics—Electricity—Heat—Mechanics—Optical Physics—Linac Radiation 2—Nuclear Radiation 2—Applied Radiation 2—Quantum Electronics 3— Electromagnetics 3—Time and Frequency 3 —Laboratory Astrophysics3—Cryo- 3 genics . -

Performance Evaluation of a Thz Pulsed Imaging System: Point Spread Function, Broadband Thz Beam Visualization and Image Reconstruction
applied sciences Article Performance Evaluation of a THz Pulsed Imaging System: Point Spread Function, Broadband THz Beam Visualization and Image Reconstruction Marta Di Fabrizio 1,* , Annalisa D’Arco 2,* , Sen Mou 2, Luigi Palumbo 3, Massimo Petrarca 2,3 and Stefano Lupi 1,4 1 Physics Department, University of Rome ‘La Sapienza’, P.le Aldo Moro 5, 00185 Rome, Italy; [email protected] 2 INFN-Section of Rome ‘La Sapienza’, P.le Aldo Moro 2, 00185 Rome, Italy; [email protected] (S.M.); [email protected] (M.P.) 3 SBAI-Department of Basic and Applied Sciences for Engineering, Physics, University of Rome ‘La Sapienza’, Via Scarpa 16, 00161 Rome, Italy; [email protected] 4 INFN-LNF, Via E. Fermi 40, 00044 Frascati, Italy * Correspondence: [email protected] (M.D.F.); [email protected] (A.D.) Abstract: Terahertz (THz) technology is a promising research field for various applications in basic science and technology. In particular, THz imaging is a new field in imaging science, where theories, mathematical models and techniques for describing and assessing THz images have not completely matured yet. In this work, we investigate the performances of a broadband pulsed THz imaging system (0.2–2.5 THz). We characterize our broadband THz beam, emitted from a photoconductive antenna (PCA), and estimate its point spread function (PSF) and the corresponding spatial resolution. We provide the first, to our knowledge, 3D beam profile of THz radiation emitted from a PCA, along its propagation axis, without the using of THz cameras or profilers, showing the beam spatial Citation: Di Fabrizio, M.; D’Arco, A.; intensity distribution. -
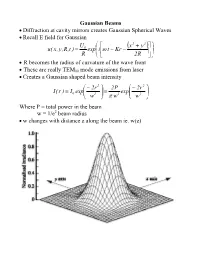
Gaussian Beams • Diffraction at Cavity Mirrors Creates Gaussian Spherical
Gaussian Beams • Diffraction at cavity mirrors creates Gaussian Spherical Waves • Recall E field for Gaussian U ⎛ ⎡ x2 + y2 ⎤⎞ 0 ⎜ ( ) ⎟ u( x,y,R,t ) = exp⎜i⎢ω t − Kr − ⎥⎟ R ⎝ ⎣ 2R ⎦⎠ • R becomes the radius of curvature of the wave front • These are really TEM00 mode emissions from laser • Creates a Gaussian shaped beam intensity ⎛ − 2r 2 ⎞ 2P ⎛ − 2r 2 ⎞ I( r ) I exp⎜ ⎟ exp⎜ ⎟ = 0 ⎜ 2 ⎟ = 2 ⎜ 2 ⎟ ⎝ w ⎠ π w ⎝ w ⎠ Where P = total power in the beam w = 1/e2 beam radius • w changes with distance z along the beam ie. w(z) Measurements of Spotsize • For Gaussian beam important factor is the “spotsize” • Beam spotsize is measured in 3 possible ways • 1/e radius of beam • 1/e2 radius = w(z) of the radiance (light intensity) most common laser specification value 13% of peak power point point where emag field down by 1/e • Full Width Half Maximum (FWHM) point where the laser power falls to half its initial value good for many interactions with materials • useful relationship FWHM = 1.665r1 e FWHM = 1.177w = 1.177r 1 e2 w = r 1 = 0.849 FWHM e2 Gaussian Beam Changes with Distance • The Gaussian beam radius of curvature with distance 2 ⎡ ⎛π w2 ⎞ ⎤ R( z ) = z⎢1 + ⎜ 0 ⎟ ⎥ ⎜ λz ⎟ ⎣⎢ ⎝ ⎠ ⎦⎥ • Gaussian spot size with distance 1 2 2 ⎡ ⎛ λ z ⎞ ⎤ w( z ) = w ⎢1 + ⎜ ⎟ ⎥ 0 ⎜π w2 ⎟ ⎣⎢ ⎝ 0 ⎠ ⎦⎥ • Note: for lens systems lens diameter must be 3w0.= 99% of power • Note: some books define w0 as the full width rather than half width • As z becomes large relative to the beam asymptotically approaches ⎛ λ z ⎞ λ z w(z) ≈ w ⎜ ⎟ = 0 ⎜ 2 ⎟ ⎝π w0 ⎠ π w0 • Asymptotically light -

Optics of Gaussian Beams 16
CHAPTER SIXTEEN Optics of Gaussian Beams 16 Optics of Gaussian Beams 16.1 Introduction In this chapter we shall look from a wave standpoint at how narrow beams of light travel through optical systems. We shall see that special solutions to the electromagnetic wave equation exist that take the form of narrow beams – called Gaussian beams. These beams of light have a characteristic radial intensity profile whose width varies along the beam. Because these Gaussian beams behave somewhat like spherical waves, we can match them to the curvature of the mirror of an optical resonator to find exactly what form of beam will result from a particular resonator geometry. 16.2 Beam-Like Solutions of the Wave Equation We expect intuitively that the transverse modes of a laser system will take the form of narrow beams of light which propagate between the mirrors of the laser resonator and maintain a field distribution which remains distributed around and near the axis of the system. We shall therefore need to find solutions of the wave equation which take the form of narrow beams and then see how we can make these solutions compatible with a given laser cavity. Now, the wave equation is, for any field or potential component U0 of Beam-Like Solutions of the Wave Equation 517 an electromagnetic wave ∂2U ∇2U − µ 0 =0 (16.1) 0 r 0 ∂t2 where r is the dielectric constant, which may be a function of position. The non-plane wave solutions that we are looking for are of the form i(ωt−k(r)·r) U0 = U(x, y, z)e (16.2) We allow the wave vector k(r) to be a function of r to include situations where the medium has a non-uniform refractive index. -

Absorption Spectroscopy
World Bank & Government of The Netherlands funded Training module # WQ - 34 Absorption Spectroscopy New Delhi, February 2000 CSMRS Building, 4th Floor, Olof Palme Marg, Hauz Khas, DHV Consultants BV & DELFT HYDRAULICS New Delhi – 11 00 16 India Tel: 68 61 681 / 84 Fax: (+ 91 11) 68 61 685 with E-Mail: [email protected] HALCROW, TAHAL, CES, ORG & JPS Table of contents Page 1 Module context 2 2 Module profile 3 3 Session plan 4 4 Overhead/flipchart master 5 5 Evaluation sheets 22 6 Handout 24 7 Additional handout 29 8 Main text 31 Hydrology Project Training Module File: “ 34 Absorption Spectroscopy.doc” Version 06/11/02 Page 1 1. Module context This module introduces the principles of absorption spectroscopy and its applications in chemical analyses. Other related modules are listed below. While designing a training course, the relationship between this module and the others, would be maintained by keeping them close together in the syllabus and place them in a logical sequence. The actual selection of the topics and the depth of training would, of course, depend on the training needs of the participants, i.e. their knowledge level and skills performance upon the start of the course. No. Module title Code Objectives 1. Basic chemistry concepts WQ - 02 • Convert units from one to another • Discuss the basic concepts of quantitative chemistry • Report analytical results with the correct number of significant digits. 2. Basic aquatic chemistry WQ - 24 • Understand equilibrium chemistry and concepts ionisation constants. • Understand basis of pH and buffers • Calculate different types of alkalinity.