Variation in Growth of Brown-Headed Cowbird (Molothrus Ater) Nestlings and Energetic Impacts on Their Host Parents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wood Warblers of Lake County (Field Guide)
Wood of Lake County An educational wildlife pamphlet provided by the Lake County Public Resources Department Parks & Trails Division 2 The Lake County Public Resources Department, Parks & Trails Division, manages more than three dozen parks, preserves and boat ramps. Lake County park rangers lead regularly scheduled nature in some of these parks. In partnership with the Lake County hikes, bird and butterfly surveys and other outdoor adventures Water Authority, Parks & Trails also schedules guided paddling adventures. For a listing of Lake County parks and events, call 352-253-4950, email [email protected] or visit Forwww.lakecountyfl.gov/parks. more information about birds that can be seen in Lake County, or bookstores. Information on birds is also available online at the check out a field guide to birds available at many local libraries Cornell Laboratory of Ornithology, www.birds.cornell.edu. Bird watchers in Florida tend to bring a little more on their trips than their Northern peers. While the average temperature in Lake County is a mild 72°F, the summer months in Central Florida can be steamy. Outside enthusiasts are always encouraged to carry sunscreen to protect skin from sunburn, insect repellent to ward off mosquitoes and plenty of water to avoid dehydration. Sunscreen should be 15 SPF or higher and applied 20 minutes before. 3 Park rangers recommend these six popular comprehensive guides: • A Field Guide to the Birds, Eastern and Central North America (Fourth Edition, 1980, Roger Tory Peterson) • Stokes Field Guide to Birds, Eastern Region (First Edition, 1996, Donald and Lillian Stokes) • All the Birds of North America (First Edition, 1997, The American Bird Conservancy) • Field Guide to the Birds of North America (Fourth Edition, 2002, The National Geographic Society) • Focus Guide to the Birds of North America (First Edition, 2000, Kenn Kaufman) • The Sibley Guide to Birds (First Edition, 2000, David Allen Sibley) Insect repellent should contain DEET. -

Pine-Oak-Hemlock--Silviculture Institute 7/18/2017 1
Pine‐Oak‐Hemlock‐‐Silviculture Institute 7/18/2017 Predicted Responses of Wildlife to Silvicultural Treatments with special focus on Pine‐Oak‐Hemlock Types Matt Tarr Associate Extension Professor, Wildlife Specialist University of New Hampshire Cooperative Extension Northeast Silviculture Institute for Foresters June 18, 2017 What am I talking about? OUTLINE • Overview of the primary factors that determine how wildlife respond to timber harvesting • Overview of how birds, mammals, reptiles & amphibians select their habitat • Summary of expected wildlife response following application of silvicultural treatments in pine‐oak‐hemlock MY GOAL: Help you become better at predicting how wildlife will respond to the decisions you make when managing forested habitats 1 Pine‐Oak‐Hemlock‐‐Silviculture Institute 7/18/2017 When we cut trees, the response by wildlife is determined primarily by: • Plant species • Plant structure composition following the harvest • The size of the canopy opening • Whether soils are • Presence/absence of • Habitats in the dry/moist/wet special habitat features surrounding landscape “Plant structure” = presence & density of plants in each forest layer • What plant layers are present? Canopy layer >30’ Midstory layer 10-30’ Shrub layer 2-10’ Herbaceous layer 0-2’ • How dense are the plants in each layer? SPARSE INTERMEDIATE ABUNDANT/DENSE < 30% coverage 30-75% coverage > 75% coverage 2 Pine‐Oak‐Hemlock‐‐Silviculture Institute 7/18/2017 Role of plant structure in influencing wildlife use of forest stands Forest stands comprised -

Ecology, Morphology, and Behavior in the New World Wood Warblers
Ecology, Morphology, and Behavior in the New World Wood Warblers A dissertation presented to the faculty of the College of Arts and Sciences of Ohio University In partial fulfillment of the requirements for the degree Doctor of Philosophy Brandan L. Gray August 2019 © 2019 Brandan L. Gray. All Rights Reserved. 2 This dissertation titled Ecology, Morphology, and Behavior in the New World Wood Warblers by BRANDAN L. GRAY has been approved for the Department of Biological Sciences and the College of Arts and Sciences by Donald B. Miles Professor of Biological Sciences Florenz Plassmann Dean, College of Arts and Sciences 3 ABSTRACT GRAY, BRANDAN L., Ph.D., August 2019, Biological Sciences Ecology, Morphology, and Behavior in the New World Wood Warblers Director of Dissertation: Donald B. Miles In a rapidly changing world, species are faced with habitat alteration, changing climate and weather patterns, changing community interactions, novel resources, novel dangers, and a host of other natural and anthropogenic challenges. Conservationists endeavor to understand how changing ecology will impact local populations and local communities so efforts and funds can be allocated to those taxa/ecosystems exhibiting the greatest need. Ecological morphological and functional morphological research form the foundation of our understanding of selection-driven morphological evolution. Studies which identify and describe ecomorphological or functional morphological relationships will improve our fundamental understanding of how taxa respond to ecological selective pressures and will improve our ability to identify and conserve those aspects of nature unable to cope with rapid change. The New World wood warblers (family Parulidae) exhibit extensive taxonomic, behavioral, ecological, and morphological variation. -

Passerines: Perching Birds
3.9 Orders 9: Passerines – perching birds - Atlas of Birds uncorrected proofs 3.9 Atlas of Birds - Uncorrected proofs Copyrighted Material Passerines: Perching Birds he Passeriformes is by far the largest order of birds, comprising close to 6,000 P Size of order Cardinal virtues Insect-eating voyager Multi-purpose passerine Tspecies. Known loosely as “perching birds”, its members differ from other Number of species in order The Northern or Common Cardinal (Cardinalis cardinalis) The Common Redstart (Phoenicurus phoenicurus) was The Common Magpie (Pica pica) belongs to the crow family orders in various fine anatomical details, and are themselves divided into suborders. Percentage of total bird species belongs to the cardinal family (Cardinalidae) of passerines. once thought to be a member of the thrush family (Corvidae), which includes many of the larger passerines. In simple terms, however, and with a few exceptions, passerines can be described Like the various tanagers, grosbeaks and other members (Turdidae), but is now known to belong to the Old World Like many crows, it is a generalist, with a robust bill adapted of this diverse group, it has a thick, strong bill adapted to flycatchers (Muscicapidae). Its narrow bill is adapted to to feeding on anything from small animals to eggs, carrion, as small birds that sing. feeding on seeds and fruit. Males, from whose vivid red eating insects, and like many insect-eaters that breed in insects, and grain. Crows are among the most intelligent of The word passerine derives from the Latin passer, for sparrow, and indeed a sparrow plumage the family is named, are much more colourful northern Europe and Asia, this species migrates to Sub- birds, and this species is the only non-mammal ever to have is a typical passerine. -
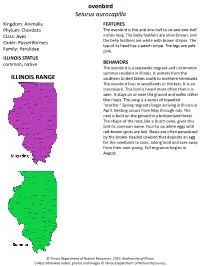
Ovenbird Seiurus Aurocapilla ILLINOIS RANGE
ovenbird Seiurus aurocapilla Kingdom: Animalia FEATURES Phylum: Chordata The ovenbird is five and one-half to six and one-half Class: Aves inches long. The body feathers are olive-brown, and Order: Passeriformes the belly feathers are white with brown stripes. The top of its head has a peach stripe. The legs are pale Family: Parulidae pink. ILLINOIS STATUS common, native BEHAVIORS The ovenbird is a statewide migrant and uncommon summer resident in Illinois. It winters from the ILLINOIS RANGE southern United States south to northern Venezuela. The ovenbird lives in woodlands or thickets. It is an insectivore. This bird is heard more often than it is seen. It stays on or near the ground and walks rather than hops. The song is a series of repeated "teacher." Spring migrants begin arriving in Illinois in April. Nesting occurs from May through July. The nest is built on the ground in a bottomland forest. The shape of the nest, like a Dutch oven, gives this bird its common name. Four to six white eggs with red-brown spots are laid. Nests are often parasitized by the brown-headed cowbird that deposits an egg for the ovenbirds to raise, taking food and care away from their own young. Fall migration begins in August. © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. adult provided by DANNYKORVES/pond5.com © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. provided by DANNYKORVES/pond5.com adult Aquatic Habitats bottomland forests Woodland Habitats bottomland forests; upland deciduous forests Prairie and Edge Habitats edge © Illinois Department of Natural Resources. -

Wood Warblers Wildlife Note
hooded warbler 47. Wood Warblers Like jewels strewn through the woods, Pennsylvania’s native warblers appear in early spring, the males arrayed in gleaming colors. Twenty-seven warbler species breed commonly in Pennsylvania, another four are rare breeders, and seven migrate through Penn’s Woods headed for breeding grounds farther north. In central Pennsylvania, the first species begin arriving in late March and early April. Louisiana waterthrush (Parkesia motacilla) and black and white warbler (Mniotilta varia) are among the earliest. The great mass of warblers passes through around mid-May, and then the migration trickles off until it ends in late May by which time the trees have leafed out, making it tough to spot canopy-dwelling species. In southern Pennsylvania, look for the migration to begin and end a few days to a week earlier; in northern Pennsylvania, it is somewhat later. As summer progresses and males stop singing on territory, warblers appear less often, making the onset of fall migration difficult to detect. Some species begin moving south as early as mid and late July. In August the majority specific habitat types and show a preference for specific of warblers start moving south again, with migration characteristics within a breeding habitat. They forage from peaking in September and ending in October, although ground level to the treetops and eat mainly small insects stragglers may still come through into November. But by and insect larvae plus a few fruits; some warblers take now most species have molted into cryptic shades of olive flower nectar. When several species inhabit the same area, and brown: the “confusing fall warblers” of field guides. -

Seiurus Aurocapilla) on VACA KEY, FLORIDA
Florida Field Naturalist 41(4):123-125, 2013. ECTOPARASITES COLLECTED FROM THE OVENBIRD (Seiurus aurocapilla) ON VACA KEY, FLORIDA LAWRENCE J. HRIBAR Florida Keys Mosquito Control District, 503 107th Street, Marathon, Florida 33050 The quill mite, Syringophiloidus seiurus (Clark) (Prostigmata: Syringophilidae) and the louse flyOrnithoctona fusciventris (Wiedemann) (Diptera: Hippoboscidae) are among the very few records of ectoparasites from the Ovenbird, Seiurus aurocapilla from Florida (Forrester and Spaulding 2003). On the 17th of November 2011, an Ovenbird was found dead outside a building on Vaca Key in Marathon, Florida (24.729984, -81.039438), apparently having collided with a plate glass window. The bird was handled and feather mites recovered and prepared for study in the same manner as were the specimens examined by Hribar and Miller (2011). Only twenty-five feather mites were recovered. Slide mounts were examined via phase contrast microscopy and then sent to a specialist for identification. Three mite species were recovered, two Proctophyllodidae (Proctophyllodes sp., Amerodectes sp.) and one Trouessartiidae. Unfortunately no specimens were readily identifiable to species. One female mite was identified as Proctophyllodes sp. Females of this genus are very difficult to identify to species, however,Proctophyllodes breviquadratus Atyeo and Braasch is known from Ovenbirds (Atyeo and Braasch 1966). One male and three female Amerodectes were not identifiable to species and may represent an undescribed species. Amerodectes mites are found on a variety of birds in the New World, viz., Apodiformes: Apodidae; Passeriformes: Cardinalidae, Emberizidae, Furnariidae, Hirundinidae, Icteridae, Parulidae, Thraupidae,and Turdidae (Valim and Hernandes 2010). The two male and two female Trouessartia mites appear to be conspecific with mites found on Ovenbirds in Alberta, Canada, and also represent an undescribed species (H. -

Display Behavior of Ovenbirds (Seiurus Aurocapillus) I
W&m Bull., 92(3), 1980, pp. 312-329 DISPLAY BEHAVIOR OF OVENBIRDS (SEIURUS AUROCAPILLUS) I. NON-SONG VOCALIZATIONS M. Ross LEIN Although detailed investigations of single displays have furnished in- sights into their proximate causation and adaptive roles in regulating social behavior (Smith 1977, Sebeok 1977), they contribute relatively little to our understanding of the evolution of animal communication at a somewhat higher level. Smith (1969a) and Moynihan (1970) pioneered consideration of the evolutionary pressures shaping the total display repertoire of a species. However, such studies are hampered by a paucity of information about the size and composition of repertoires of most animals, since de- tailed inventories are time-consuming and are frequently by-passed by workers studying single displays or small portions of a repertoire. Gen- eralizations are therefore based on a few species whose display behavior is known in sufficient detail, and their validity depends on how well ad- ditional studies conform to the patterns found. During investigations of behavior of wood warblers (Parulidae) I ob- tained information on the display repertoires of various species. In this paper I describe the non-song vocalizations of breeding Ovenbirds (Seiurus aurocupillus) and suggest some of the functional aspects of vocal com- munication and the evolutionary pressures that possibly have shaped its display repertoire. The songs of males and visual displays will be dealt with in subsequent papers. The Ovenbird is an excellent subject for behavioral investigation. It is widely distributed and abundant throughout the wooded regions of North America, occupying open woodlands, generally with little underbrush but with an abundance of fallen leaves, logs and rocks (Bent 1953, Griscom and Sprunt 1957). -

2020 National Bird List
2020 NATIONAL BIRD LIST See General Rules, Eye Protection & other Policies on www.soinc.org as they apply to every event. Kingdom – ANIMALIA Great Blue Heron Ardea herodias ORDER: Charadriiformes Phylum – CHORDATA Snowy Egret Egretta thula Lapwings and Plovers (Charadriidae) Green Heron American Golden-Plover Subphylum – VERTEBRATA Black-crowned Night-heron Killdeer Charadrius vociferus Class - AVES Ibises and Spoonbills Oystercatchers (Haematopodidae) Family Group (Family Name) (Threskiornithidae) American Oystercatcher Common Name [Scientifc name Roseate Spoonbill Platalea ajaja Stilts and Avocets (Recurvirostridae) is in italics] Black-necked Stilt ORDER: Anseriformes ORDER: Suliformes American Avocet Recurvirostra Ducks, Geese, and Swans (Anatidae) Cormorants (Phalacrocoracidae) americana Black-bellied Whistling-duck Double-crested Cormorant Sandpipers, Phalaropes, and Allies Snow Goose Phalacrocorax auritus (Scolopacidae) Canada Goose Branta canadensis Darters (Anhingidae) Spotted Sandpiper Trumpeter Swan Anhinga Anhinga anhinga Ruddy Turnstone Wood Duck Aix sponsa Frigatebirds (Fregatidae) Dunlin Calidris alpina Mallard Anas platyrhynchos Magnifcent Frigatebird Wilson’s Snipe Northern Shoveler American Woodcock Scolopax minor Green-winged Teal ORDER: Ciconiiformes Gulls, Terns, and Skimmers (Laridae) Canvasback Deep-water Waders (Ciconiidae) Laughing Gull Hooded Merganser Wood Stork Ring-billed Gull Herring Gull Larus argentatus ORDER: Galliformes ORDER: Falconiformes Least Tern Sternula antillarum Partridges, Grouse, Turkeys, and -

Volume 2E - Revised Baseline Ecological Risk Assessment Hudson River Pcbs Reassessment
PHASE 2 REPORT FURTHER SITE CHARACTERIZATION AND ANALYSIS VOLUME 2E - REVISED BASELINE ECOLOGICAL RISK ASSESSMENT HUDSON RIVER PCBS REASSESSMENT NOVEMBER 2000 For U.S. Environmental Protection Agency Region 2 and U.S. Army Corps of Engineers Kansas City District Book 2 of 2 Tables, Figures and Plates TAMS Consultants, Inc. Menzie-Cura & Associates, Inc. PHASE 2 REPORT FURTHER SITE CHARACTERIZATION AND ANALYSIS VOLUME 2E- REVISED BASELINE ECOLOGICAL RISK ASSESSMENT HUDSON RIVER PCBs REASSESSMENT RI/FS CONTENTS Volume 2E (Book 1 of 2) Page TABLE OF CONTENTS ........................................................ i LIST OF TABLES ........................................................... xiii LIST OF FIGURES ......................................................... xxv LIST OF PLATES .......................................................... xxvi EXECUTIVE SUMMARY ...................................................ES-1 1.0 INTRODUCTION .......................................................1 1.1 Purpose of Report .................................................1 1.2 Site History ......................................................2 1.2.1 Summary of PCB Sources to the Upper and Lower Hudson River ......4 1.2.2 Summary of Phase 2 Geochemical Analyses .......................5 1.2.3 Extent of Contamination in the Upper Hudson River ................5 1.2.3.1 PCBs in Sediment .....................................5 1.2.3.2 PCBs in the Water Column ..............................6 1.2.3.3 PCBs in Fish .........................................7 -

Brown-Headed Cowbird: Agent of Extermination?
Brown-headed Cowbird: agcnt of cxtcrmination? by Harold Mayfield Hooded Warblerfeeding a youngcowbird. Photo by Alvin E. S taffan. HEN A BEGINNINGbirdwatcher finds a maudlinsuperficiality. cowbird egg or large voraciousyoung in The customary reassurances have much the nest of a small songbird, he invariably truth in them. But theyare not the wholetruth. reactswith indignation, if not violence.But the Relationshipsin nature are often complex-- more sophisticatednaturalist reassureshim, sometimesso complex we do not claim to pointing out that there should be no causefor understandthem fully -- but everythingis not alarm, that nature's ways are sometimes goingwell with everyliving creature.In nature inscrutable but these birds would not be here if there are losersas well as winners.At any they couldn't live together. momentwe are likely to be looking at a select group of survivors.What about those that fell The prevailing mood among naturalists is by the wayside? that wild creatures are usually secure among themselves. The obvious dangers are often Extinction also is a reality of nature. inconsequential.and apparent enemies may Naturalists are acutely aware of the intrusion actually be friends in disguise.This senseof of man, and theseare oftenso gross as to make dynamicequilibrium is embodiedin the famil- us forgetfulof changingstress buried deeperin iar phrase "balance of nature." Also serious the fabric of nature. Changeis inevitable.Some studentsof biology have acquired a deep dis- changes are abrupt and dramatic. Those trust of sentimentality.and the prevailingview wrought by man are almost instantaneousin may have an element of backlash against nature's scale of time, but so are those caused Volume 31, Number 2 107 by naturalcataclysms --volcanoes, forest fires, How the cowbird operates tidal waves, and hurricanes. -

Osmoregulation and Adaptive Radiation in the Ovenbird 20, 799–805 Genus Cinclodes (Passeriformes: Furnariidae)
Functional Blackwell Publishing Ltd Ecology 2006 Osmoregulation and adaptive radiation in the ovenbird 20, 799–805 genus Cinclodes (Passeriformes: Furnariidae) P. SABAT,*†‡ K. MALDONADO,* M. CANALS* and C. MARTINEZ DEL RIO§ *Departamento de Ciencias Ecológicas, Facultad de Ciencias, Universidad de Chile, Casilla, 653, Santiago, Chile, ‡Center for Advanced Studies in Ecology and Biodiversity, Facultad de Ciencias Biológicas Pontificia Universidad Católica de Chile, Santiago, Chile, and §Department of Zoology and Physiology, University of Wyoming, Laramie, USA Summary 1. The genus Cinclodes is unique among passerines because it includes two species that can be considered marine/coastal and also includes several species that inhabit fresh- water streams or that shift habitats between terrestrial/fresh water and marine habitats. The Cinclodes clade satisfies two criteria of an adaptive radiation: it is monophyletic and it experienced recent speciation accompanied by rapid phenotypic diversification. 2. We focused on the osmoregulatory traits of five Cinclodes species to determine if the clade also satisfies the criterion of adaptive phenotype–environment correlation that characterizes adaptive radiations. We used the δ13C of tissues to estimate reliance on a marine diet. We predicted that δ13C would be positively correlated with the renal traits responsible for urine concentration (relative kidney size, fraction of the kidney comprising medulla, and number of medullary cones per unit of kidney mass). 3. Our analyses confirmed these hypotheses. We concluded that Cinclodes satisfies the adaptive phenotype–environment correlation criterion. Cinclodes seems to represents an example of an avian adaptive radiation in osmoregulatory function. Key-words: Carbon isotopes, kidney, marine birds, renal function, salt Functional Ecology (2006) 20, 799–805 doi: 10.1111/j.1365-2435.2006.01176.x for the relative scarcity of marine species among Introduction passerines.