Influence of Spawning Ground Dynamics on the Long
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Squid and Octopus
Sector Improvement Profile: Squid and Octopus More than half (52%) of global production is yellow- or red-rated, indicating that improvements are needed, and 45% is status unknown. Our improvement efforts prioritize: • The 27% of global squid and octopus production that is red-rated; and • The 39% within the scope of Sustainable Fisheries Partnership’s Target 75 Initiative. Red-rated Species Source country Argentine shortfin squid Argentina Common octopus Mexico, Portugal, Spain Common squids nei Indonesia, Thailand Japanese flying squid Japan Mexican four-eyed octopus Mexico Octopuses nei Indonesia, Mauritania, Morocco, Philippines Various squids nei China, India The Monterey Bay Aquarium’s Seafood Watch Program ratings are based on specific location and production method information and make exceptions for specific brands. For detailed information, visit the Seafood Watch recommendations on squid and octopus. FIP or AIP Where available, the links below lead to detailed information about FIPs listed on FisheryProgress.org. Species Source country Day Octopus Madagascar California Two-Spot Octopus Mexico Giant Pacific Octopus Japan Japanese Flying Squid China Jumbo Squid Mexico, Peru Mitre Squid China Red octopus Chile, Mexico Octopus China The Seafood Certification & Ratings Collaboration unites five global seafood certification and ratings programs working together to coordinate our tools and increase our impact so that more seafood producers move along a clear path toward environmental sustainability and social responsibility. Learn -

Learning About Our Favourite Squid Species
Cephalopod Science Investigations LEARNING ABOUT OUR FAVOURITE SQUID SPECIES By Cushla Dromgool-Regan Eimear Manning & Anna Quinn www.EXPLORERS.ie The Explorers Education Programme is funded by the Marine Institute Explorers Education Programme engage with primary schools, teachers and children, creating marine leaders and ocean champions. The Explorers Education Programme team provides engaging activities, resources and support for teachers, children and the education network, delivering ocean literacy to primary schools. We aim to inspire children and educators to learn about our marine and maritime identity and heritage, as well as making informed and responsible decisions regarding the ocean and its resources. We communicate about the ocean in a meaningful way, increasing the awareness and understanding of our marine biodiversity, the environment, as well as the opportunities and social benefits of our ocean wealth. To help inspire children learning about the ocean, we have developed a series of teaching materials and resources about Squid! Check out our Explorers books: Cephalopod Science Investigations – Learning about Squid 101; My CSI Squid Workbook. Also, see our interactive film: Cephalopod Science Investigations – Learning about Squid 101 and Dissection. For more information about our Squid series see www.explorers.ie CEPHALOPOD SCIENCE INVESTIGATIONS LEARNING ABOUT OUR FAVOURITE SQUID SPECIES AUTHORS Cushla Dromgool-Regan Eimear Manning Anna Quinn PUBLISHED BY Marine Institute First published in 2021 Marine Institute, Rinville, Oranmore, Galway All or parts of the content of this publication may be reproduced without further permission for education purposes, provided the author and publisher are acknowledged. Authors: Cushla Dromgool-Regan, The Camden Education Trust; Eimear Manning, The Camden Education Trust; & Anna Quinn, Galway Atlantaquaria. -

Squids, Octopuses and Lots of Ink
Squids, octopuses and lots of ink Rodrigo B. Salvador1 & Carlo M. Cunha2 1 Staatliches Museum für Naturkunde Stuttgart; Stuttgart, Germany. Eberhard Karls Universität Tübingen; Tübingen, Germany. Email: [email protected] 2 Museu de Zoologia da Universidade de São Paulo; São Paulo, Brazil. Email: [email protected] Splatoon was recently released (second the class Cephalopoda belongs to the phylum quarter of 2015) for the Wii U, receiving a warm Mollusca. welcome by Nintendo fans (it’s nigh unthinkable Cephalopoda is a group that contains a vast for the company to launch a new IP like this) and array of marine animals. Besides squids and generating a flood of fan art on the Internet. The octopuses, it counts with cuttlefish, nautiloids game is a third-person shooter with ink instead and the fossil belemnites and ammonoids. of bullets. It features two races, inklings (the Today, cephalopods are found everywhere in playable one) and octarians (the enemies), and the sea, from the polar regions to the tropics and revolves around the fierce dispute against each from the surface to depths over 5,000 m. There other. (In multiplayer though, its inkling against are over 800 known living species of inkling.) Inklings and octarians (especially the cephalopods, but the fossil record counts with elite soldiers called “octolings”) are based, more than 17,000 species (Boyle & Rodhouse, respectively, on squids and octopuses (Fig. 1), 2005; Rosenberg, 2014). two of the most awesome kinds of animals out The class appeared over 450 million years there. ago during the late Cambrian, the first period of These animals are mollusks, and, more the Paleozoic era (Boyle & Rodhouse, 2005; specifically, cephalopods. -
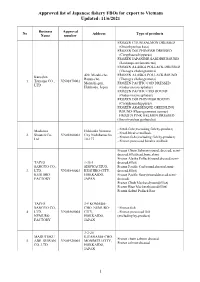
Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

Wgceph Report 2018
ICES WGCEPH REPORT 2018 ECOSYSTEM PROCESSES AND DYNAMICS STEERING GROUP ICES CM 2018/EPDSG:12 REF. SCICOM Interim Report of the Working Group on Cephalopod Fisheries and Life History (WGCEPH) 5-8 June 2018 Pasaia, San Sebastian, Spain International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer H. C. Andersens Boulevard 44–46 DK-1553 Copenhagen V Denmark Telephone (+45) 33 38 67 00 Telefax (+45) 33 93 42 15 www.ices.dk [email protected] Recommended format for purposes of citation: ICES. 2019. Interim Report of the Working Group on Cephalopod Fisheries and Life History (WGCEPH), 5–8 June 2018, Pasaia, San Sebastian, Spain. ICES CM 2018/EPDSG:12. 194 pp. https://doi.org/10.17895/ices.pub.8103 The material in this report may be reused for non-commercial purposes using the recommended citation. ICES may only grant usage rights of information, data, imag- es, graphs, etc. of which it has ownership. For other third-party material cited in this report, you must contact the original copyright holder for permission. For citation of datasets or use of data to be included in other databases, please refer to the latest ICES data policy on ICES website. All extracts must be acknowledged. For other re- production requests, please contact the General Secretary. The document is a report of an Expert Group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the views of the Council. © 2019 International Council for the Exploration of the Sea ICES WGCEPH REPORT 2018 | i Contents Executive summary ............................................................................................................... -

Prey Consumption and Feeding Habits of Three Baleen Whale Species In
PreyPrey consumptionconsumption andand feedingfeeding habitshabits ofof threethree baleenbaleen whalewhale speciesspecies inin thethe westernwestern NorthNorth PacificPacific (PICES(PICES // W3)W3) Tsutomu TAMURA and Kenji KONISHI 1 OutlineOutline ofof JARPNJARPN IIII --11-- Research area : From 140E to 170E Research period : From May to September Research objectives 1. Feeding ecology of whales and ecosystem studies 2. Monitoring environmental pollutants in cetaceans and the marine ecosystem 3. Stock structure 2 OutlineOutline ofof JARPNJARPN IIII --22-- Common minke whale (Balaenoptera acutorostrata) Body length 7 m Body weight 5 t 220 inds./year Sei whale (Balaenoptera borealis) Body length 14 m Body weight 22 t 100 inds./year Bryde’s whale (Balaenoptera edeni) Body length 13 m Body weight 16 t 50 inds./year Sperm whale (Physeter macrocephalus) Body length Male: 15m, Female: 11m 10 inds./year Body weight Male: 40t , Female: 18t 3 OutlineOutline ofof JARPNJARPN IIII --33-- Measurement of body weight Identification of stomach contents Sampling of stomach contents Measurement of stomach contents The relative prey composition (%) by weight of each prey species (RW) RW = (Wi / Wall ) × 100 Wi = the weight of contents containing prey group i W = the total weight of contents analyzed. all 4 PreyPrey speciesspecies ofof baleenbaleen whalewhale speciesspecies Copepods Krill Japanese anchovy Chub mackerel Pacific saury Japanese flying squid (Neocalanus spp.) (Euphausia pacifica) (Engraulis japonicus) (Scomber japonicus) (Colorabis saira) (Tadarodes -

Squid Supply, Demand, and Market of Japan
DECEMBER 2018 SQUID SUPPLY, DEMAND, AND MARKET OF JAPAN Sunee C. Sonu U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service West Coast Region TABLE OF CONTENTS Page LIST OF TABLES ........................................... ii LIST OF FIGURES ......................................... iii EXECUTIVE SUMMARY ........................................ iv INTRODUCTION .............................................. 1 FISHERY ................................................... 2 IMPORTS .................................................. 13 Trade Barriers......................................... 14 COLD STORAGE HOLDINGS .................................... 23 SUPPLY ................................................... 25 EXPORTS .................................................. 27 DEMAND ................................................... 30 WHOLESALE PRICES ......................................... 31 REFERENCES ............................................... 39 i LIST OF TABLE Page 1. World landings of squid and cuttlefish by major countries, 1950-2016....................................... 4 2. Japan’s landings of Ommastrephid squid, 2014-2016.......... 5 3. Landings of Todarodes pacificus, and total squid and cuttlefish landings by world and by Japan, 1950-2016....... 7 4. Japan’s landings of Ommastrephes bartrami, 1974-2016....... 8 5. Japan’s landings of Dosidicus gigas, 1991-2016............ 10 6. Japan’s landings of Nototodarus sloani, and Illex argentinus, 1980-2016.................................... -

Conference 2015 Program
CEPHALOPOD INTERNATIONAL ADVISORY COUNCIL CONFERENCE 2015 PROGRAM NOVEMBER 6-14 Hakodate, Japan CIAC 2015 PROGRAM BOOK Cephalopod International Advisory Council Conference 2015 平成27年 国際頭足類諮問委員会函館会議 Recent Advances in Cephalopod Science November 6-14, 2015 Hakodate, Japan CIAC 2015 CIAC 2015 Table of contents Welcome messages ……………………………………………………………………... 2 Organizers and sponsors ……………………………………………………………... 5 General information ……………………………………………………………………... 6 Restaurants and bars near the Hakodate Kokusai Hotel ………………………...…… 7 Map of conference venue ……………………………………….……………………. 10 Notes …………………………………………………………………………………….. 11 Conference timetable …………………………………………………………………….. 12 Pre-symposium workshops and mini-workshops ………………………………………….. 13 Symposium program November 10 …………………………………………………………………….. 16 November 11 ………………………………………………………………..…… 19 November 12 ………………………………………………………………..…… 22 November 13 …………………………………………………………………..… 23 November 14 …………………………………………………………………….. 27 Posters ……………………………………….……………………………………………. 30 List of registrants …………….……………………….………….…………….……... 45 Index of presenters …………………….………………………………………………... 58 CIAC 2015 Welcome to the 2015 CIAC Conference On behalf of the Cephalopod International Advisory Council and Organizing Committee, it is my honor to welcome you to the beautiful and historic city of Hakodate for the 2015 CIAC conference. The theme for this year’s conference - Recent Advances in Cephalopod Science – emphasizes the rapid progress and growing interest in the field of cephalopod research. Since the last -

Chinese Japanese Flying Squid (JFS) Fisheries Improvement Scoping Report
Chinese Japanese Flying Squid (JFS) Fisheries Improvement Scoping Report December 2018 Ocean Outcomes (O2) Contacts: Songlin Wang, China Program Director Qing Fang, Consultant Dr. Jocelyn Drugan, Analytics Team Director and Fishery Scientist Rich Lincoln, Founder and Senior Advisor www.oceanoutcomes.org 1 Chinese JFS Fishery Improvement Scoping Report: October 2018 TABLE OF CONTENTS 1. BACKGROUND .............................................................................................................................................. 4 1.1 OVERVIEW OF FISHERY PRE-ASSESSMENT .......................................................................................................................... 4 1.2 OVERVIEW OF FIP SCOPING .................................................................................................................................................... 5 2. STOCK AND FISHERY DESCRIPTION ...................................................................................................... 6 2.1 SPECIES AND STOCK .................................................................................................................................................................. 6 2.2 FISHERY OVERVIEW .................................................................................................................................................................. 9 2.2.1 Location ............................................................................................................................................................................ -

Invertebrados Marinos
Invertebrados marinos Español English Français abanico de mar sea fan gorgone acalefo azul shift arms jellyfish rhizostome acantocéfalos acanthocephalia acanthocéphales actinia roja red sea anemone actinie pourpre actinia solar daisy anemone anémone solaire almeja clam palourde ameba ameba amibe anélidos annelida annélides anémona anemone anémone anémona cerianto cylinder anemone cérianthe de Méditerranée anémona de mar sea anemone anémone de mer anémona de mar común snakelocks anemone anémone de mer anémona incrustante amarilla yellow encrusting sea anemone anémone encroûtante jaune anfioxo amphioxus amphioxus arca Noah's ark arche de Noé arenícola sandworm arénicole ascidia roja red sea squirt ascidie rouge aurelia common jellyfish amélie avispa de mar sea wasp guêpe de mer berberecho cockle coque bígaro periwinkle bigorneau bivalvo bivalve bivalve bogavante European lobster homard braquiolaria brachiolaria brachiolaria braquiópodos brachiopoda brachiopodes braquiuros brachyura brachyoures briozoos bryozoa bryozoaires buccino whelk buccin calamar squid calmar calamar común common squid encornet calamar de El Cabo chokker squid calmar du Cap calamar de Patagonia Patagonian squid calmar patagon calamar estrella seven-star flying squid encornet étoile calamar gigante giant squid calmar géant calamar gigante jumbo flying squid encornet géant calamar rojo broadtail shorftin squid encornet rouge calamar rojo argentino Argentine shortfin squid encornet rouge argentin camarón monegasco Monaco shrimp crevette monégasque cangrejo crab crabe Español -

Japanese Ying Squid Japan
Japanese ying squid Todarodes pacicus Japan: North and Northwest Pacic Jig, Unassociated purse seine, Bottom trawls, Trap net Fisheries Standard Version F2 December 5, 2016 Ernest Chen, Consulting Researcher Disclaimer Seafood Watch® strives to have all Seafood Reports reviewed for accuracy and completeness by external scientists with expertise in ecology, sheries science and aquaculture. Scientic review, however, does not constitute an endorsement of the Seafood Watch® program or its recommendations on the part of the reviewing scientists. Seafood Watch® is solely responsible for the conclusions reached in this report. 1 Table of Contents About. .Seafood . Watch. 3. Guiding. .Principles . 4. Summary. 5. Final. Seafood. .Recommendations . 6. Introduction. 8. Assessment. 11. Criterion. .1: . .Impacts . on. the. species. under. assessment. .11 . .............. Criterion. .2: . .Impacts . on. other. species. .16 . .............. Criterion. .3: . .Management . .Effectiveness . .20 . .............. Criterion. .4: . .Impacts . on. the. habitat. .and . .ecosystem . .27 . .............. Acknowledgements. 31. References. 32. Appendix. A:. Extra. .By . Catch. .Species . 36. 2 About Seafood Watch Monterey Bay Aquarium’s Seafood Watch® program evaluates the ecological sustainability of wild-caught and farmed seafood commonly found in the United States marketplace. Seafood Watch® denes sustainable seafood as originating from sources, whether wild-caught or farmed, which can maintain or increase production in the long-term without jeopardizing the structure or function of aected ecosystems. Seafood Watch® makes its science-based recommendations available to the public in the form of regional pocket guides that can be downloaded from www.seafoodwatch.org. The program’s goals are to raise awareness of important ocean conservation issues and empower seafood consumers and businesses to make choices for healthy oceans. -

Export Opportunities for U.S. Squid
NOAA Technical Memorandum NMFS NOVEMBER 1989 EXPORT OPPORTUNITIES FOR US. SQUID Sunee C. Sonu NOAA-TM-N MFS-SW R-022 U.S. DEPARTMENT OF COMMERCE National Oceanic & Atmospheric Administration National Marine Fisheries Service Southwest Region NOAA Technical Memorandum NMFS The National Oceanic and Atmospheric Administration (NOAA), organized in 1970, has evolved into an agency which establishes national policies and manages and conserves our oceanic, coastal, and atmospheric resources. An organizational element within NOAA, the Office of Fisheries is responsible for fisheries policy and the direction of the National Marine Fisheries Service (NMFS). In addition to its formal publications, the NMFS uses the NOAA Technical Memorandum series to issue informal scientific and technical publications when complete formal review and editorial processing are not appropriate or feasible. Documents within this series, however, reflect sound professional work and may be referenced in the formal scientific and technical literature. NOAA Technical Memorandum NMFS This TM rekb used for documentatirm and timely communication d preliminary results. interim rsports. OI special pufposa information;and have not received mefd rsview. editorid mntrd. or detailed editing. NOVEMBER 1989 EXPORT OPPORTUNITIES FOR U.S. SQUID Sunee C. Sonu Southwest Region National Marine Fisheries Service, NOAA Terminal Island, California 90731 NOM-TM-NMFS-SWR-022 U.S. DEPARTMENT OF COMMERCE Robert A. Mosbacher, Secretary National Oceanic and Atmospheric Administration John A. Knauss, Under Secretary for Oceans and Atmosphere National Marine Fisheries Service James E. Douglas, Jr., Acting Assistant Administrator for Fisheries TABLE OF CONTENTS Page LIST OF TABLES ............... ii LIST OF FIGURES . iv LIST OF APPENDICES ............. V EXECUTIVE SUMMARY ............