Consensus Statement for the Prevention and Management of Pain in the Newborn
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ADESIONE ALLE TERAPIE a LUNGO TERMINE Problemi E Possibili Soluzioni
ADESIONE ALLE TERAPIE A LUNGO TERMINE problemi e possibili soluzioni Published by the World Health Organization in 2003 under the title Adherence to long term therapies: Evidence for action © World Health Organization 2003 The Director-General of the World Health Organization has granted translation rights for an edition in Italian to Critical Medicine Publishing s.r.l., which is solely responsible for the italian edition. Edizione italiana a cura di E. Grossi Tutti i diritti riservati. Nessuna parte di questa pubblicazione può essere riprodotta o trasmessa in alcuna forma o con alcun mezzo, compresa la registrazione o le fotocopie, senza il permesso scritto dell’editore. © 2006 - Critical Medicine Publishing Editore 00143 Roma - Via G. Squarcina, 3 - Tel. 06.51951.1 www.cmpedizioni.it Realizzazione grafica e stampa: Istituto Arti Grafiche Mengarelli - Roma ISBN 88-88415-25-4 Questa pubblicazione, realizzata con la collaborazione di Bracco S.p.A. è offerta in omaggio ai sigg. Medici Indice Sezione I Disegnare lo scenario 19 Capitolo I Definire il concetto di adesione ai trattamenti 21 Capitolo II Le dimensioni del problema della scarsa adesione ai trattamenti 27 Capitolo III Come può la scarsa adesione ai trattamenti interessare politici e manager della sanità? 31 SEZIONE II Migliorare l’adesione ai trattamenti: una guida per i paesi 37 Capitolo IV Le lezioni apprese 39 Capitolo V Verso la soluzione 51 Capitolo VI Come può il miglioramento dell’adesione ai trattamenti tradursi in benefici economici e per la salute? 71 SEZIONE III Review -

Provocative Discography 4 5 Irina Melnik , Richard Derby , and Ray M
Provocative Discography 4 5 Irina Melnik , Richard Derby , and Ray M. Baker Key Points • Technical challenges, potential complications, and • Discography is an invasive diagnostic procedure interpretation mistakes can be avoided with proper not intended to be an initial screening examination selection of patients, including favorable psycho- due to associated potential risk to a patient. logical pro fi ling, use of sterile technique, intravenous • It is a con fi rmatory test, which can reveal the true and intradiscal antibiotics, judicious use of sedation, source of pain and thus leads to precise and effec- and good technical training of a practitioner. tive treatment as well as might help patients to avoid • Emerging alternative approaches including anes- unnecessary surgical interventions. thetic discography and functional discography are • The value of the test is not only in providing morpho- gaining attention, as well as noninvasive MRI spec- logic characteristics of the disc structure and degrees troscopy and other imaging tests, as an attempt to of internal annular disc disrupture but also in providing provide similar clinical information without putting unique clinical information by potentially evoking patients at a potential short- or long-term risk. patients typical/concordant pain and con fi rming a speci fi c level of the painful disc. • As a provocative test, discography is liable to false- positive results, which can be potentially avoided Introduction by adherence to strict operational standards and interpretation criteria, including pain ³ 7/10, pres- Discography was introduced in the 1940s to diagnose her- sure <50 psi a.o. , concordant pain, ³ grade 3 annu- niation and internal annular disruption of the lumbar and lar tear, volume £ 3.5 mL, and the presence of a subsequently cervical and thoracic intervertebral discs [ 1, 2 ] . -

Q2 2020 Trend Highlights
2020 Mid-Year THETHE STSTAATETE OFOF THETHE HOTHOT 100 100 TOPTOP 1010 SELECT HIGHLIGHTS Compositional and Industry Trends for the Billboard Hot 100 Top 10 The State of the Hot 100 Top 10 takes an in-depth look at Q1 and Q2 2020's compositional and industry-related trends for the Billboard Hot 100 Top 10. What follows are a few select highlights from the report. SONGS..............................................................................................................................................................PAGE 2 PERFORMING ARTISTS.........................................................................................................................PAGE 3 SONGWRITERS...........................................................................................................................................PAGE 6 PRODUCERS.................................................................................................................................................PAGE 10 RECORD LABELS.......................................................................................................................................PAGE 13 #1 SPOTLIGHT..............................................................................................................................................PAGE 16 2019 VS. 2020 COMPOSITIONAL TRENDS.............................................................................PAGE 20 Data is for songs that charted in the Billboard Hot 100 Top 10 and excludes holiday songs. Data related to compositional characteristics -

Geriatric Rheumatology: a Comprehensive Approach Encourages You to Think from the Older Patient’S Perspective
Geriatric Rheumatology wwwwwwwwwwwwwwwwwwwwww Yuri Nakasato • Raymond L. Yung Editors Geriatric Rheumatology A Comprehensive Approach Editors Yuri Nakasato Raymond L. Yung Sanford Health Systems Department of Internal Medicine Fargo, ND, USA University of Michigan [email protected] Ann Arbor, Michigan, USA [email protected] ISBN 978-1-4419-5791-7 e-ISBN 978-1-4419-5792-4 DOI 10.1007/978-1-4419-5792-4 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2011928680 © Springer Science+Business Media, LLC 2011 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with respect to the material contained herein. -

Human Rights Constitutionalism in Japan and Asia
Page 1 Human Rights Constitutionalism in Japan and Asia The Writings of Lawrence W. Beer Lawrence Ward Beer was born in Portland, Oregon on May 11, 1932. In 1966 he received the Ph.D. degree from the University of Washington, Seattle. In over fifty years of studying the constitutional law and politics of Japan and other Asian countries, he has written and lectured extensively on human rights law (e.g., Freedom of Expression in Japan, 1984). He taught at the University of Colorado, Boulder, 1966–1982, and was F.M. Kirby Professor of Civil Rights, Lafayette College, 1982–1997. Lawrence W. Beer has chaired the Committee on Asian Law of the Association for Asian Studies and the World Association of Law Professors of the World Peace through Law Center. He received the Distinguished Asianist Award of the Mid-Atlantic Association for Asian Studies in 2003. In retirement, he lives with his wife Keiko in Boulder, Colorado, USA. Less noticed in the West than wars, terrorism and economic trends has been the historic develop- ment since World War II of constitutional government and law in Asia. Lawrence W. Beer has been a close observer of Asian linkages among law, politics, culture, and national security issues for over fifty years. His perspectives have been refined during long residence in Asia, especially Japan, by substantial friendly interactions with Asian legal scholars, judges and attorneys involved in the world of human rights constitutional law. This volume, which will be widely welcomed by students and researchers, brings together a selection of Lawrence W. Beer’s many works previously published in diverse venues, but no longer easily accessible. -

Chiropractic and Spinal Research
TABLE OF CONTENTS Dedication ......................................................................................................7 Introduction ...................................................................................................9 Is Chiropractic A Treatment For Disease? ................................................ 11 Chiropractic And Musculoskeletal Conditions.........................................12 Cost-Benefit of Chiropractic ......................................................................13 Acknowledgement .......................................................................................14 Spinal Care and its Effects on Human Physiology in Sickness and in Health...........................................................................................................17 Allergies, Sinus Trouble ..............................................................................18 Anorexia Nervosa ........................................................................................19 Arnold-Chiari Malformation .....................................................................20 Arthritis/Reversal of Arthritis ...................................................................20 Asthma .........................................................................................................23 Attention Deficit Disorder and Hyperactivity ..........................................29 Autism, Behavioral And Learning Disorders ...........................................34 Bed-Wetting .................................................................................................38 -

Treatment for Acute Pain: an Evidence Map Technical Brief Number 33
Technical Brief Number 33 R Treatment for Acute Pain: An Evidence Map Technical Brief Number 33 Treatment for Acute Pain: An Evidence Map Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov Contract No. 290-2015-0000-81 Prepared by: Minnesota Evidence-based Practice Center Minneapolis, MN Investigators: Michelle Brasure, Ph.D., M.S.P.H., M.L.I.S. Victoria A. Nelson, M.Sc. Shellina Scheiner, PharmD, B.C.G.P. Mary L. Forte, Ph.D., D.C. Mary Butler, Ph.D., M.B.A. Sanket Nagarkar, D.D.S., M.P.H. Jayati Saha, Ph.D. Timothy J. Wilt, M.D., M.P.H. AHRQ Publication No. 19(20)-EHC022-EF October 2019 Key Messages Purpose of review The purpose of this evidence map is to provide a high-level overview of the current guidelines and systematic reviews on pharmacologic and nonpharmacologic treatments for acute pain. We map the evidence for several acute pain conditions including postoperative pain, dental pain, neck pain, back pain, renal colic, acute migraine, and sickle cell crisis. Improved understanding of the interventions studied for each of these acute pain conditions will provide insight on which topics are ready for comprehensive comparative effectiveness review. Key messages • Few systematic reviews provide a comprehensive rigorous assessment of all potential interventions, including nondrug interventions, to treat pain attributable to each acute pain condition. Acute pain conditions that may need a comprehensive systematic review or overview of systematic reviews include postoperative postdischarge pain, acute back pain, acute neck pain, renal colic, and acute migraine. -

Communal Pastoral Counselling: Culturally Gifted Care-Giving in Times of Family Pain—A Vhavenda Perspective
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Unisa Institutional Repository COMMUNAL PASTORAL COUNSELLING: CULTURALLY GIFTED CARE-GIVING IN TIMES OF FAMILY PAIN—A VHAVENDA PERSPECTIVE BY DEMBE REUBEN PHASWANA Submitted in accordance with the requirements for the degree of DOCTOR OF THEOLOGY in the subject PRACTICAL THEOLOGY at the UNIVERSITY OF SOUTH AFRICA PROMOTER: DR. M E HESTENES NOVEMBER 2008 ……………… a DECLARATION I Dembe Reuben Phaswana, student number: 0421 588 – 5 declare that COMMUNAL PASTORAL COUNSELLING: CULTURALLY GIFTED CARE-GIVING IN TIMES OF FAMILY PAIN—A VHAVENDA PERSPECTIVE is my own work and that all other sources that I have used or quoted have been indicated and acknowledged by means of complete references. __________________________ _____________________ DR PHASWANA _____________ DATE ______________ b ACKNOWLEDGEMENTS This thesis is the illustration of communal endeavour. Without the communal involvement I wouldn’t have started and submitted this thesis. I thank God All Mighty, the Father of our Lord Jesus Christ. He is big, and I am small. It reminds me of a trip of an elephant and mouse crossing the bridge. After crossing the bridge the mouse commented, “Did you hear how we shake that bridge?” The elephant agreed that they did shake the bridge, while the real shaking was done by the elephant. In this project God is the one that did the real shaking. “Soli Deo Gloria!” My thanks go to my promoter Dr. M.E. Hestenes. He gave me encouragement from the first day. As I keep on writing and rewriting he was reading and rereading with encouraging comments. -
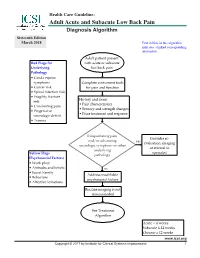
Adult Acute and Subacute Low Back Pain Diagnosis Algorithm Sixteenth Edition March 2018 Text in Blue in This Algorithm Indicates a Linked Corresponding Annotation
Health Care Guideline: Adult Acute and Subacute Low Back Pain Diagnosis Algorithm Sixteenth Edition March 2018 Text in blue in this algorithm indicates a linked corresponding annotation. Adult patient present Red Flags for with acute or subacute Underlying low back pain Pathology • Cauda equina symptoms Complete assessment tools • Cancer risk for pain and function • Spinal infection risk • Fragility fracture History and exam: risk • Pain characteristics • Unrelenting pain • Sensory and strength changes • Progressive • Prior treatment and response neurologic deficit • Trauma Incapacitating pain Consider re- and/or advancing yes evaluation, imaging neurologic symptoms or other or referral to underlying Yellow Flags specialist pathology (Psychosocial Factors) • Work place • Attitudes and beliefs no • Sociał/family Address modifiable • Behaviors psychosocial factors • Affective/emotions Routine imaging is not recommended See Treatment Algorithm Acute < 4 weeks Subacute 4-12 weeks Chronic ≥ 12 weeks www.icsi.org Copyright © 2017 by Institute for Clinical Systems Improvement 1 Adult Acute and Subacute Low Back Pain Sixteenth Edition/March 2018 Yellow Flags Treatment Algorithm (Psychosocial Factors) Acute or subacute • Work place low back pain • Attitudes and beliefs diagnosis • Social /family • Behaviors • Affective/emotions Routine imaging is not recommended Establish treatment goals using shared decision-making: • Patient goals • Clinical goals • Patient barriers • Psychosocial factors Develop a Treatment Plan Patient education Self-care Non-pharmacologic -

Pain: Psychological Perspectives 1 Thomas Hadjistavropoulos and Kenneth D
Pain PSYCHOLOGICAL PERSPECTIVES Edited by Thomas Hadjistavropoulos Kenneth D. Craig PAIN Psychological Perspectives PAIN Psychological Perspectives Edited by Thomas Hadjistavropoulos University of Regina Kenneth D. Craig University of British Columbia LAWRENCE ERLBAUM ASSOCIATES, PUBLISHERS 2004 Mahwah, New Jersey London Copyright © 2004 by Lawrence Erlbaum Associates, Inc. All rights reserved. No part of this book may be reproduced in any form, by photostat, microform, retrieval system, or any other means, without the prior written permission of the publisher. Lawrence Erlbaum Associates, Inc., Publishers 10 Industrial Avenue Mahwah, New Jersey 07430 Cover design by Sean Sciarrone Library of Congress Cataloging-in-Publication Data Pain : psychological perspectives / edited by Thomas Hadjistavropoulos, Kenneth D. Craig. p. cm. Includes bibliographical references and index. ISBN 0-8058-4299-3 (alk. paper) 1. Pain—Psychological aspects. I. Hadjistavropoulos, Thomas. II. Craig, Kenneth D., 1937– BF515.P29 2003 152.1¢824—dc21 2003052862 CIP Books published by Lawrence Erlbaum Associates are printed on acid-free paper, and their bindings are chosen for strength and durability. Printed in the United States of America 10987654321 We dedicate this volume to those who mean the most to us: Heather, Nicholas, and Dimitri —T. H. Sydney, Kenneth, Alexandra, and Jamie —K. D. C. Contents Contributors ix Preface xi An Introduction to Pain: Psychological Perspectives 1 Thomas Hadjistavropoulos and Kenneth D. Craig 1 The Gate Control Theory: Reaching for the Brain 13 Ronald Melzack and Joel Katz 2 Biopsychosocial Approaches to Pain 35 Gordon J. G. Asmundson and Kristi D. Wright 3 Pain Perception, Affective Mechanisms, and Conscious Experience 59 C. Richard Chapman 4 Social Influences and the Communication of Pain 87 Thomas Hadjistavropoulos, Kenneth D. -

“You Went There for the People and Went There for the Bands”
“You went there for the people and went there for the bands” The Sandringham Hotel – 1980 to 1998 Brendan Paul Smyly Doctor of Philosophy University of Western Sydney August 2010 2 Acknowledgements I offer thanks to my supervisors, principally Dr Diana Blom whose knowledge, good advise and enthusiasm for a broad array of topics was invaluable for the length of this study. To Dr Greg Noble, a serendipitous choice who provided great counsel at the oddest hours and introduced me to whole vistas of academic view, thank you. Also to Professor Michael Atherton who offered unwavering support (along with some choice gigs) over the years of my engagement with UWS, many thanks. I offer very special thanks to all my colleagues from the Music Department at UWS. Ian Stevenson and Dr Sally Macarthur, thank you for your faith and support. To John Encarnacao, who was also an interviewee for the project, your advice and enthusiasm for the project has been invaluable and saw me through some difficult months, as has your friendship, thank you. To Mitchell Hart, your knowledge and willingness to help with myriad tasks whenever asked is greatly appreciated. My time as a student in this department will be fondly remembered due to you people being there. Many thanks to the administrative staff of the School of Communication Arts, particularly Robin Mercer for candidature funding and travel advice, and Tracy Mills at the Office of Research Service for your prompt and valuable help. Above all, I wish to thank Darinca Blajic. Thank you for your unfailing love, support and wonderful food. -

Coping Or Acceptance: What to Do About Chronic Pain?
Pain 105 (2003) 197–204 www.elsevier.com/locate/pain Coping or acceptance: what to do about chronic pain? Lance M. McCracken*, Chris Eccleston Pain Management Unit, Royal National Hospital for Rheumatic Diseases and University of Bath, Bath BA1 1RL, UK Received 9 January 2003; received in revised form 5 March 2003; accepted 5 May 2003 Abstract Research and treatment of chronic pain over the past 20 or more years have tended to focus on patient coping as the primary behavioral contribution to adjustment. The purpose of the present study was to compare a coping approach to chronic pain with a different behavioral approach referred to as acceptance of chronic pain. These approaches were compared in terms of their ability to predict distress and disability in a sample of patients seeking treatment for chronic pain. Subjects were 230 adults assessed at a university pain management center. All patients completed the coping strategies questionnaire and the chronic pain acceptance questionnaire among other standard measures. Results showed that coping variables were relatively weakly related to acceptance of pain and relatively unreliably related to pain adjustment variables. On the other hand, acceptance of chronic pain was associated with less pain, disability, depression and pain-related anxiety, higher daily uptime, and better work status. Regression analyses examined the independent contributions of coping and acceptance to key adjustment indicators in relation to chronic pain. Results from these analyses demonstrated that acceptance of pain repeatedly accounted for more variance than coping variables. q 2003 International Association for the Study of Pain. Published by Elsevier B.V.