Anti-Hiv Agents: a Step Towards Future
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Enzyme Inducers Efavirenz and Tipranavir/Ritonavir on the Pharmacokinetics of the HIV Integrase Inhibitor Dolutegravir
Eur J Clin Pharmacol (2014) 70:1173–1179 DOI 10.1007/s00228-014-1732-8 CLINICAL TRIAL Effects of enzyme inducers efavirenz and tipranavir/ritonavir on the pharmacokinetics of the HIV integrase inhibitor dolutegravir Ivy Song & Julie Borland & Shuguang Chen & Phyllis Guta & Yu Lou & David Wilfret & Toshihiro Wajima & Paul Savina & Amanda Peppercorn & Stephen Castellino & David Wagner & Louise Hosking & Michael Mosteller & Justin P.Rubio & Stephen C. Piscitelli Received: 7 May 2014 /Accepted: 11 August 2014 /Published online: 23 August 2014 # The Author(s) 2014. This article is published with open access at Springerlink.com Abstract study due to increases in alanine aminotransferase that were Purpose Dolutegravir (DTG) is an unboosted, integrase in- considered related to TPV/r. Co-administration with EFV hibitor for the treatment of HIV infection. Two studies evalu- resulted in decreases of 57, 39 and 75 % in DTG AUC(0–τ), ated the effects of efavirenz (EFV) and tipranavir/ritonavir Cmax and Cτ, respectively. Co-administration with TPV/r re- (TPV/r) on DTG pharmacokinetics (PK) in healthy subjects. sulted in decreases of 59, 46 and 76 % in DTG AUC(0–τ), Cmax Methods The first study was an open-label crossover where and Cτ, respectively. 12 subjects received DTG 50 mg every 24 hours (q24h) for Conclusions Given the reductions in exposure and PK/ 5 days, followed by DTG 50 mg and EFV 600 mg q24h for pharmacodynamic relationships in phase II/III trials, DTG 14 days. The second study was an open-label crossover where should be given at an increased dose of 50 mg twice daily 18 subjects received DTG 50 mg q24h for 5 days followed by when co-administered with EFV or TPV/r, and alternative TPV/r 500/200 mg every 12 hours (q12h) for 7 days and then regimens without inducers should be considered in integrase DTG 50 mg q24h and TPV/r 500/200 mg q12h for a further inhibitor-resistant patients. -

HIV Tropism Testing for Maraviroc Response
Lab Management Guidelines V1.0.2021 HIV Tropism Testing for Maraviroc Response MOL.TS.185.A v1.0.2021 Introduction HIV tropism testing for maraviroc response is addressed by this guideline. Procedures addressed The inclusion of any procedure code in this table does not imply that the code is under management or requires prior authorization. Refer to the specific Health Plan's procedure code list for management requirements. Procedures addressed by this Procedure codes guideline HIV-1 Tropism Phenotyping 87999 HIV-1 Tropism Genotyping, Common 87901 HIV-1 Tropism Genotyping, Other 87906 What is HIV tropism testing for Maraviroc response Definition HIV tropism testing is used to help determine an individual's response to maraviroc (Selzentry®). Maraviroc is only effective against CCR5-tropic HIV-1. Human immunodeficiency virus (HIV) HIV replicates itself in humans by infecting T-cells with CD4 receptors (often called CD4 cells). HIV-1 enters the CD4 cell by binding one of two cell surface co-receptors: CCR5 or CXCR4.1,2 Tropism classifications Tropism is the ability of HIV-1 virus to use one or both of these co-receptors. There are three main tropism classifications:3 o CCR5 tropism (R5-tropic) — HIV-1 virus that only infects cells with the CCR5 co-receptor. o CXCR4 tropism (X4-tropic) — HIV-1 virus that only infects cells with the CXCR4 co-receptor. © 2020 eviCore healthcare. All Rights Reserved. 1 of 5 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Lab Management Guidelines V1.0.2021 o Dual or mixed tropism (D/M-tropic) — HIV-1 virus populations that can use either co-receptor to infect cells. -

Revised 4/1/2021 GEORGIA MEDICAID FEE-FOR-SERVICE HIV
GEORGIA MEDICAID FEE-FOR-SERVICE HIV-AIDS PA SUMMARY Preferred (may not be all inclusive) Non-Preferred Abacavir generic Abacavir/lamivudine/zidovudine generic Abacavir/lamivudine generic Aptivus (tipranavir) Complera (emtricitabine/rilpivirine/tenofovir disoproxil Atazanavir capsules generic fumarate) Atripla (efavirenz/emtricitabine/tenofovir disoproxil Crixivan (indinavir) fumarate) Biktarvy (bictegravir/emtricitabine/tenofovir Delstrigo (doravirine/lamivudine/tenofovir disoproxil alafenamide) fumarate) Cimduo (lamivudine/tenofovir disoproxil fumarate) Fuzeon (enfuvirtide) Descovy (emtricitabine/tenofovir alafenamide) Intelence (etravirine) Dovato Invirase (saquinavir) Edurant (rilpivirine)* Lexiva (fosamprenavir) Efavirenz tablets generic Nevirapine extended-release generic Emtriva (emtricitabine) Norvir Powder (ritonavir) Epivir solution (lamivudine) Pifeltro (doravirine) Evotaz (atazanavir/cobicistat)* Reyataz Powder (atazanavir) Genvoya (elvitegravir/cobicistat/emtricitabine/ Ritonavir tablets generic tenofovir alafenamide) Isentress and Isentress HD (raltegravir)* Rukobia (fostemsavir) Juluca (dolutegravir/rilpivirine) Selzentry (maraviroc) Kaletra (lopinavir/ritonavir) Stavudine generic^ Stribild (elvitegravir/cobicistat/emtricitabine/ tenofovir Lamivudine generic disoproxil fumarate) Symfi (efavirenz 600 mg/lamivudine/tenofovir Lamivudine/zidovudine generic disoproxil fumarate) Symfi Lo (efavirenz 400 mg/lamivudine/tenofovir Nevirapine immediate-release tablets generic disoproxil fumarate) Norvir (ritonavir) Temixys (lamivudine/tenofovir -
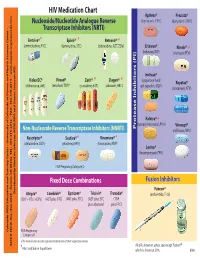
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (Tipranavir, TPV) (Darunavir, DRV) Transcriptase Inhibitors (NRTI)
HIV Medication Chart Aptivus® Prezista® Nucleoside/Nucleotide Analogue Reverse (tipranavir, TPV) (darunavir, DRV) Transcriptase Inhibitors (NRTI) Emtriva®* Epivir® * Retrovir® * (emtricitabine, FTC) (lamivudine, 3TC) (zidovudine, AZT, ZDV) Crixivan® Norvir® * (indinavir, IDV) (ritonavir, RTV) Invirase® Videx EC® Viread® Zerit® Ziagen® * * (saquinavir hard Reyataz® (didanosine, ddl) (tenofovir,TDF)* (stavudine, d4T) (abacavir, ABC) gel capsules, SQV) (atazanavir, ATV) Kaletra® * (lopinavir/ritonavir, LPV/r) Viracept® Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI) Inhibitors (PI) Protease (nelfinavir, NFV) Rescriptor® Sustiva®* Viramune® * (delavirdine, DLV) (efavirenz, EFV) (nevirapine, NVP) Lexiva® (fosamprenavir, FPV) FDA Pregnancy Category D Fixed Dose Combinations Fusion Inhibitors Fuzeon® Developed by MeriLou Johnson, MeriLou by Developed Johnson, MSW, and Steven MPA MD Atripla® Combivir® Epzicom® Trizivir® Truvada® (enfuvirtide,T-20) (TDF+FTC+EFV) (AZT plus 3TC) (ABC plus 3TC) (AZT plus 3TC (TDF plus abacavir) plus FTC) Colorado AIDS Education and Training Center • University of Colorado at Denver and Health Sciences Center • Center and Health Sciences Denver at of Colorado • University Center Training and AIDS Education Colorado FDA Pregnancy Category D ®The brands listed are the registered trademarks of their respective owners. 4200 E. Ave., Ninth TEL: A089 • Denver, Box 80262 CO 303-315-2512 • FAX: • 303-315-2514 • www.mpaetc.org/colorado.htm All pills shown in actual size except Fuzeon® *Also available in liquid form. which is shown at 50%. 8/06 Medication Schedule Name______________________________ Date___________ Number of Time of day you ar to take TOTAL Name of Medication pills to take this medicine Food Interactions Side Effects number of each time pills each day With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ With Food Without Food ❑ ❑ Discontinued Medications or Formulations Helpful Hints: • Refill prescriptions before you run out. -

Truvada (Emtricitabine / Tenofovir Disoproxil)
Pre-exposure Prophylaxis (2.3) HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Recommended dose in HIV-1 uninfected adults: One tablet TRUVADA safely and effectively. See full prescribing information (containing 200 mg/300 mg of emtricitabine and tenofovir for TRUVADA. disoproxil fumarate) once daily taken orally with or without food. (2.3) TRUVADA® (emtricitabine/tenofovir disoproxil fumarate) tablets, for oral use Recommended dose in renally impaired HIV-uninfected Initial U.S. Approval: 2004 individuals: Do not use TRUVADA in HIV-uninfected individuals if CrCl is below 60 mL/min. If a decrease in CrCl is observed in WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH uninfected individuals while using TRUVADA for PrEP, evaluate STEATOSIS, POST-TREATMENT ACUTE EXACERBATION OF potential causes and re-assess potential risks and benefits of HEPATITIS B, and RISK OF DRUG RESISTANCE WITH USE OF continued use. (2.4) TRUVADA FOR PrEP IN UNDIAGNOSED HIV-1 INFECTION -----------------------DOSAGE FORMS AND STRENGTHS------------------- See full prescribing information for complete boxed warning. Tablets: 200 mg/300 mg, 167 mg/250 mg, 133 mg/200 mg, and 100 Lactic acidosis and severe hepatomegaly with steatosis, mg/150 mg of emtricitabine and tenofovir disoproxil fumarate . (3) including fatal cases, have been reported with the use of nucleoside analogs, including VIREAD, a component of TRUVADA. (5.1) --------------------------------CONTRAINDICATIONS----------------------------- TRUVADA is not approved for the treatment of chronic Do not use TRUVADA for pre-exposure prophylaxis in individuals with hepatitis B virus (HBV) infection. Severe acute unknown or positive HIV-1 status. TRUVADA should be used in exacerbations of hepatitis B have been reported in patients HIV-infected patients only in combination with other antiretroviral coinfected with HIV-1 and HBV who have discontinued agents. -

Download Article PDF/Slides
New Antiretrovirals in Development: Reprinted from The PRN Notebook,™ june 2002. Dr. James F. Braun, Editor-in-Chief. Tim Horn, Executive Editor. Published in New York City by the Physicians’ Research Network, Inc.,® John Graham Brown, Executive Director. For further information and other articles The View in 2002 available online, visit http://www.PRN.org All rights reserved. © june 2002. Roy “Trip” Gulick, md, mph Associate Professor of Medicine, Weill Medical College of Cornell University Director, Cornell Clinical Trials Unit, New York, New York Summary by Tim Horn Edited by Scott Hammer, md espite the fact that 16 antiretro- tiviral activity of emtricitabine was estab- Preliminary results from two random- virals are approved for use in the lished, with total daily doses of 200 mg or ized studies—FTC-302 and FTC-303—were United States, there is an indis- more producing the greatest median viral reported by Dr. Charles van der Horst and putable need for new anti-hiv com- load suppression: 1.72-1.92 log. Based on his colleagues at the 8th croi, held in Feb- pounds that have potent and these data, a once-daily dose of 200 mg ruary 2001 in Chicago (van der Horst, durable efficacy profiles, unique re- was selected for further long-term clinical 2001). FTC-302 was a blinded comparison sistance patterns, patient-friendly dosing study. “This is what we’re looking forward of emtricitabine and lamivudine, both in schedules, and minimal toxicities. To pro- to with emtricitabine,” commented Dr. combination with stavudine (Zerit) and vide prn with a glimpse of drugs current- Gulick. -

Review CCR5 Antagonists: Host-Targeted Antivirals for the Treatment of HIV Infection
Antiviral Chemistry & Chemotherapy 16:339–354 Review CCR5 antagonists: host-targeted antivirals for the treatment of HIV infection Mike Westby* and Elna van der Ryst Pfizer Global R&D, Kent, UK *Corresponding author: Tel: +44 1304 649876; Fax: +44 1304 651819; E-mail: [email protected] The human chemokine receptors, CCR5 and suggest that these compounds have a long plasma CXCR4, are potential host targets for exogenous, half-life and/or prolonged CCR5 occupancy, which small-molecule antagonists for the inhibition of may explain the delay in viral rebound observed HIV-1 infection. HIV-1 strains can be categorised by following compound withdrawal in short-term co-receptor tropism – their ability to utilise CCR5 monotherapy studies. A switch from CCR5 to (CCR5-tropic), CXCR4 (CXCR4-tropic) or both (dual- CXCR4 tropism occurs spontaneously in approxi- tropic) as a co-receptor for entry into susceptible mately 50% of HIV-infected patients and has been cells. CCR5 may be the more suitable co-receptor associated with, but is not required for, disease target for small-molecule antagonists because a progression. The possibility of a co-receptor natural deletion in the CCR5 gene preventing its tropism switch occurring under selection pressure expression on the cell surface is not associated by CCR5 antagonists is discussed. The completion with any obvious phenotype, but can confer of ongoing Phase IIb/III studies of maraviroc, resistance to infection by CCR5-tropic strains – the aplaviroc and vicriviroc will provide further insight most frequently sexually-transmitted strains. into co-receptor tropism, HIV pathogenesis and The current leading CCR5 antagonists in clinical the suitability of CCR5 antagonists as a potent development include maraviroc (UK-427,857, new class of antivirals for the treatment of HIV Pfizer), aplaviroc (873140, GlaxoSmithKline) and infection. -

Product Monograph for CELSENTRI
PRODUCT MONOGRAPH PrCELSENTRI maraviroc Tablets 150 and 300 mg CCR5 antagonist ViiV Healthcare ULC 245, boulevard Armand-Frappier Laval, Quebec H7V 4A7 Date of Revision: July 05, 2019 Submission Control No: 226222 © 2019 ViiV Healthcare group of companies or its licensor. Trademarks are owned by or licensed to the ViiV Healthcare group of companies. Page 1 of 60 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION.........................................................3 SUMMARY PRODUCT INFORMATION ........................................................................3 INDICATIONS AND CLINICAL USE..............................................................................3 CONTRAINDICATIONS ...................................................................................................3 WARNINGS AND PRECAUTIONS..................................................................................4 ADVERSE REACTIONS....................................................................................................9 DRUG INTERACTIONS ..................................................................................................19 DOSAGE AND ADMINISTRATION..............................................................................28 OVERDOSAGE ................................................................................................................31 ACTION AND CLINICAL PHARMACOLOGY ............................................................31 STORAGE AND STABILITY..........................................................................................36 -

PATIENT INFORMATION STRIBILD® (STRY-Bild) (Elvitegravir, Cobicistat
PATIENT INFORMATION STRIBILD® (STRY-bild) (elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate) tablets Important: Ask your healthcare provider or pharmacist about medicines that should not be taken with STRIBILD. For more information, see the section “What should I tell my healthcare provider before taking STRIBILD?” What is the most important information I should know about STRIBILD? STRIBILD can cause serious side effects, including: • Worsening of Hepatitis B infection. If you have hepatitis B virus (HBV) infection and take STRIBILD, your HBV may get worse (flare-up) if you stop taking STRIBILD. A “flare-up” is when your HBV infection suddenly returns in a worse way than before. o Do not run out of STRIBILD. Refill your prescription or talk to your healthcare provider before your STRIBILD is all gone. o Do not stop taking STRIBILD without first talking to your healthcare provider. o If you stop taking STRIBILD, your healthcare provider will need to check your health often and do blood tests regularly for several months to check your HBV infection. Tell your healthcare provider about any new or unusual symptoms you may have after you stop taking STRIBILD. See “What are the possible side effects of STRIBILD?” for more information about side effects. What is STRIBILD? STRIBILD is a prescription medicine that is used without other antiretroviral medicines to treat Human Immunodeficiency Virus-1 (HIV-1) in people 12 years of age and older: • who have not received anti-HIV-1 medicines in the past, or • to replace their current anti-HIV-1 medicines: o in people who have been on the same anti-HIV-1 medicine regimen for at least 6 months, and o who have an amount of HIV-1 in their blood (this is called “viral load”) that is less than 50 copies/mL, and o have never failed past HIV-1 treatment. -

The CCR5-Delta32 Variant Might Explain Part of the Association Between COVID-19 and the Chemokine-Receptor Gene Cluster
medRxiv preprint doi: https://doi.org/10.1101/2020.11.02.20224659; this version posted November 4, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. The CCR5-delta32 variant might explain part of the association between COVID-19 and the chemokine-receptor gene cluster 1,8,12* 1,8* 2,8,9,10,11 Juan Gómez, Elías Cuesta-Llavona, Guillermo M. Albaiceta, Marta 3 4,8,9,12 2,8,10,11 García-Clemente, Carlos López-Larrea, Laura Amado-Rodríguez, Inés 8,10 3 3 1,8 1,8 López-Alonso, Tamara Hermida, Ana I. EnrÍquez, Helena Gil , Belén Alonso, 1,8 1 1,8,9,12 Sara Iglesias, Beatriz Suarez-Alvarez,4,8,12 Victoria Alvarez, Eliecer Coto *These authors contributed equally to this work. 1 Genética Molecular, Hospital Universitario Central Asturias, Oviedo, Spain. 2 Unidad de Cuidados Intensivos Cardiológicos, Hospital Universitario Central Asturias, Oviedo, Spain. 3 Neumología, Hospital Universitario Central Asturias, Oviedo, Spain. 4 Inmunología, Hospital Universitario Central Asturias, Oviedo, Spain. 5 Urgencias, Hospital Universitario Central Asturias, Oviedo, Spain. 8 Instituto de Investigación Sanitaria del Principado deAsturias, ISPA, Oviedo, Spain. 9 Universidad de Oviedo, Oviedo, Spain. 10 CIBER-Enfermedades Respiratorias. Instituto de Salud Carlos III. Madrid, Spain. 11 Instituto Universitario de Oncología del Principado de Asturias. Oviedo, Spain. 12 Red de Investigación Renal (REDINREN), Madrid, Spain. Correspondenceto: Dr.EliecerCoto Hospital Univ. Central Asturias 33011 – Oviedo – Spain Tel. 34.985.10.55.00 Email: [email protected] 1 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

(12) Patent Application Publication (10) Pub. No.: US 2008/0241135 A1 Olson Et Al
US 20080241135A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0241135 A1 Olson et al. (43) Pub. Date: Oct. 2, 2008 (54) METHODS FOR REDUCINGVIRAL LOAD IN 60/711,528, filed on Aug. 26, 2005, provisional appli HIV-1-INFECTED PATIENTS cation No. 60/715,619, filed on Sep. 9, 2005. (75) Inventors: William C. Olson, Ossining, NY Publication Classification (US); Paul J. Maddon, Scarsdale, NY (US); Daniel C. Pevear, (51) Int. Cl. Medford, MA (US); Robert J. A 6LX 39/395 (2006.01) Israel, Suffern, NY (US); Jose D. A6IP 43/00 (2006.01) Murga, Rosedale, NY (US) (52) U.S. Cl. ..................................................... 424/133.1 Correspondence Address: (57) ABSTRACT COOPER & DUNHAM, LLP 1185 AVENUE OF THE AMERICAS This method provides a method for reducing HIV-1 viral load NEW YORK, NY 10036 in an HIV-1-infected human subject which comprises admin istering to the subject at a predefined interval effective HIV-1 (73) Assignee: Progenics Pharmaceuticals, Inc. viral load-reducing doses of (a) a humanized antibody desig nated PRO 140, or of (b) an anti-CCR5 receptor monoclonal (21) Appl. No.: 11/894,762 antibody. This invention also provides a method for inhibiting in a human Subject the onset or progression of an HIV-1- (22) Filed: Aug. 20, 2007 associated disorder, the inhibition of which is effected by inhibiting fusion of HIV-1 to CCR5"CD4" target cells in the Related U.S. Application Data Subject. This invention also provides a method for treating a subject infected with HIV-1 comprising administering to the (63) Continuation of application No. -

Guidelines for the Use of Antiretroviral Agents in Adults and Adolescent Living With
Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV Developed by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC) How to Cite the Adult and Adolescent Guidelines: Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/ AdultandAdolescentGL.pdf. Accessed [insert date] [insert page number, table number, etc. if applicable] It is emphasized that concepts relevant to HIV management evolve rapidly. The Panel has a mechanism to update recommendations on a regular basis, and the most recent information is available on the HIVinfo Web site (http://hivinfo.nih.gov). What’s New in the Guidelines? August 16, 2021 Hepatitis C Virus/HIV Coinfection • Table 18 of this section has been updated to include recommendations regarding concomitant use of fostemsavir or long acting cabotegravir plus rilpivirine with different hepatitis C treatment regimens. June 3, 2021 What to Start • Since the release of the last guidelines, updated data from the Botswana Tsepamo study have shown that the prevalence of neural tube defects (NTD) associated with dolutegravir (DTG) use during conception is much lower than previously reported. Based on these new data, the Panel now recommends that a DTG-based regimen can be prescribed for most people with HIV who are of childbearing potential. Before initiating a DTG-based regimen, clinicians should discuss the risks and benefits of using DTG with persons of childbearing potential, to allow them to make an informed decision.