Titre De La These
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Fisheries of Chile
The Fisheries of Chile UNITED STATES DEPARTMENT OF THE INTERIOR FISH AND WILDLIFE SERVICE BUREAU OF COMMERCIAL FISHERIES Circular 234 UNITED STATES DEPARTMENT OF THE IXTERIOI Stewart L. Udall, Secretary John A. Carvel', Jr., Under Secretary Stanley A. Cain, Assistant Secretary for Fi~h and W ildlife and Park.' FISH AND WILDLIFE SERVICE, Clarence F. PauL~kP , COmIll1s.~lonP/' BUREAU OF COMMERCIAL FISHERIES, Donald L. :\IcK rnan, Director The Fisheries of Ch ile By SIDNEY SHAPIRO Circular 234 Washington, D.C. Novern.ber 1965 CONTENTS Page 1 2 3 3 4 4 5 5 7 7 7 7 7 8 8 8 8 8 9 9 9 9 10 10 13 14 Iii The Fisheries of Chile By SIDNEY SHAPIRO, Forelgn Fisheries SpeClallst Bureau of Commercial F ishenes, Washmgton, D.C. ABSTRACT Trends and developments in the Chilean fishenes are discussed, wIth speCl 1 emphasis given to the expanding fish reduction mdustry. WIthm a few years Chile has become one of the world's largest producers and exporters of fish meal and od. Information is also presented on other exportable products, mamly shnmp and plated lobster (langostino), and on segments of the mdustry that produce for domestic consumption. INTRODUCTION the case in many Latin American countnes, fish supplies are Irregular; marketmg and Attention is being focused on the Chilean distribution facilities are inadequdte, espe fisheries because of the recent rapid increase cially in rural areas; and the low purchas.ng in production of fish meal. In Latin America, power of many people limits purchases. Con the Chilean fisheries are second only to those sumption of fishery products IS highest lr1 of Peru, the world's leading fishing nation in the more densely populated central reglOn. -
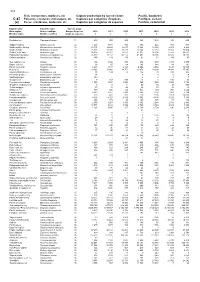
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

© Iccat, 2007
A5 By-catch Species APPENDIX 5: BY-CATCH SPECIES A.5 By-catch species By-catch is the unintentional/incidental capture of non-target species during fishing operations. Different types of fisheries have different types and levels of by-catch, depending on the gear used, the time, area and depth fished, etc. Article IV of the Convention states: "the Commission shall be responsible for the study of the population of tuna and tuna-like fishes (the Scombriformes with the exception of Trichiuridae and Gempylidae and the genus Scomber) and such other species of fishes exploited in tuna fishing in the Convention area as are not under investigation by another international fishery organization". The following is a list of by-catch species recorded as being ever caught by any major tuna fishery in the Atlantic/Mediterranean. Note that the lists are qualitative and are not indicative of quantity or mortality. Thus, the presence of a species in the lists does not imply that it is caught in significant quantities, or that individuals that are caught necessarily die. Skates and rays Scientific names Common name Code LL GILL PS BB HARP TRAP OTHER Dasyatis centroura Roughtail stingray RDC X Dasyatis violacea Pelagic stingray PLS X X X X Manta birostris Manta ray RMB X X X Mobula hypostoma RMH X Mobula lucasana X Mobula mobular Devil ray RMM X X X X X Myliobatis aquila Common eagle ray MYL X X Pteuromylaeus bovinus Bull ray MPO X X Raja fullonica Shagreen ray RJF X Raja straeleni Spotted skate RFL X Rhinoptera spp Cownose ray X Torpedo nobiliana Torpedo -

Monthly Highlights
Monthly Highlights No. 2 / 2021 In this issue As of 2021, the Monthly Highlights include Bulgaria among surveyed countries. According to data collected by EUMOFA from 13 EU Member States, in November 2020 striped venus and whelk together accounted for 12% of the total Contents first-sales value of the “Bivalves and other molluscs and aquatic invertebrates” commodity group. First sales in Europe Striped venus (Italy, Spain) and whelk From 2018 to 2020, the price of live, fresh, or chilled (Belgium, France, Netherlands) mussels imported in the EU from Norway fluctuated from 2,04 to 7,09 EUR/kg. In 2020, both price and Extra-EU imports volume exhibited a downward trend. Weekly average EU import prices of selected products from Over the last four years, German consumers spent selected countries of origin the most for a kilogram of fresh cod, (18,00 EUR/kg on average) compared to France Consumption Fresh cod in Germany, France, and the (16,80 EUR/kg) and the Netherlands (16,30 EUR/kg). Netherlands In 2019, the EU imports of fisheries and aquaculture products from South Africa accounted for over EUR Case studies 295 million and 80.597 tonnes. Hake, squid, and Fisheries and aquaculture in South Africa fishmeal constitute the bulk share of EU imports. Horse mackerel in the EU The largest market for horse mackerel exported by the EU is Egypt. In 2019, exports to the country Global highlights reached 54.000 tonnes, worth EUR 51 million, accounting for 50% of total export volume Macroeconomic context and 47% of value. Marine fuel, consumer prices, and In January 2021, the EU and Greenland concluded exchange rates negotiations for a new four-year Sustainable Fisheries Partnership Agreement (SFPA), which is the third most important agreement in place for the EU in financial terms. -

Global Standard for Responsible Supply of Marine Ingredients
IFFO RS Global Standard for Responsible Supply of Marine Ingredients Global Standard for Responsible Supply of Marine Ingredients Fishery Assessment Methodology and Template Report V2.0 Version No.: 2.0 Date: July 2017 Page 1 IFFO RS Global Standard for Responsible Supply of Marine Ingredients Chilean jack mackerel, Jurel (Trachurus murphyi) Fishery Under Assessment Chile EEZ XV-X Date December 2019 Assessor Jim Daly Application details and summary of the assessment outcome Name: Blumar and others Address: Country: Chile Zip: Tel. No.: Fax. No.: Email address: Applicant Code Key Contact: Title: Certification Body Details Name of Certification Body: SAI Global Ltd Assessment Initial/Surveillance/ Whole fish/ Assessor Name Peer Reviewer Days Re-approval By-product Jim Daly Conor Donnelly 3 SURV 1 Whole fish Assessment Period 2019 Scope Details SUBPESCA & SERNAPESCA, Chile EEZ; Management Authority (Country/State) SPRFMO International Waters Main Species Chilean jack mackerel Trachurus murphyi Fishery Location Chile EEZ VX-X Gear Type(s) Purse seine, hand-line Outcome of Assessment Chilean Jack mackerel Jurel Trachurus PASS murphyi Clauses Failed NONE Pacific Chub mackerel Caballa Scomber PASS japonicus Clauses Failed NONE Blue fathead Pez medusa Cubiceps caeruleus PASS Clauses Failed NONE PASS Snoek Sierra Thyrsites atun Clauses Failed NONE Version No.: 2.0 Date: July 2017 Page 2 Peer Review Evaluation APPROVE Recommendation PASS Assessment Determination The Northern fishery (XV-II) is mostly within the Chilean EEZ; the Central-Southern fishery (III-X) is within the Chilean EEZ and also straddles international waters. The Central-Southern fishery is used mainly for the reduction fishery for Chilean Jack mackerel. -

A Preliminary Global Assessment of the Status of Exploited Marine Fish and Invertebrate Populations
A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS June 30 2018 A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS Maria. L.D. Palomares, Rainer Froese, Brittany Derrick, Simon-Luc Nöel, Gordon Tsui Jessika Woroniak Daniel Pauly A report prepared by the Sea Around Us for OCEANA June 30, 2018 A PRELIMINARY GLOBAL ASSESSMENT OF THE STATUS OF EXPLOITED MARINE FISH AND INVERTEBRATE POPULATIONS Maria L.D. Palomares1, Rainer Froese2, Brittany Derrick1, Simon-Luc Nöel1, Gordon Tsui1, Jessika Woroniak1 and Daniel Pauly1 CITE AS: Palomares MLD, Froese R, Derrick B, Nöel S-L, Tsui G, Woroniak J, Pauly D (2018) A preliminary global assessment of the status of exploited marine fish and invertebrate populations. A report prepared by the Sea Around Us for OCEANA. The University of British Columbia, Vancouver, p. 64. 1 Sea Around Us, Institute for the Oceans and Fisheries, University of British Columbia, 2202 Main Mall, Vancouver BC V6T1Z4 Canada 2 Helmholtz Centre for Ocean Research GEOMAR, Düsternbrooker Weg 20, 24105 Kiel, Germany TABLE OF CONTENTS Executive Summary 1 Introduction 2 Material and Methods 3 − Reconstructed catches vs official catches 3 − Marine Ecoregions vs EEZs 3 − The CMSY method 5 Results and Discussion 7 − Stock summaries reports 9 − Problematic stocks and sources of bias 14 − Stocks in the countries where OCEANA operates 22 − Stock assessments on the Sea Around Us website 31 − The next steps 32 Acknowledgements 33 References 34 Appendices I. List of marine ecoregions by EEZ 37 II. Summaries of number of stock by region and 49 by continent III. -

New Zealand Fishes a Field Guide to Common Species Caught by Bottom, Midwater, and Surface Fishing Cover Photos: Top – Kingfish (Seriola Lalandi), Malcolm Francis
New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing Cover photos: Top – Kingfish (Seriola lalandi), Malcolm Francis. Top left – Snapper (Chrysophrys auratus), Malcolm Francis. Centre – Catch of hoki (Macruronus novaezelandiae), Neil Bagley (NIWA). Bottom left – Jack mackerel (Trachurus sp.), Malcolm Francis. Bottom – Orange roughy (Hoplostethus atlanticus), NIWA. New Zealand fishes A field guide to common species caught by bottom, midwater, and surface fishing New Zealand Aquatic Environment and Biodiversity Report No: 208 Prepared for Fisheries New Zealand by P. J. McMillan M. P. Francis G. D. James L. J. Paul P. Marriott E. J. Mackay B. A. Wood D. W. Stevens L. H. Griggs S. J. Baird C. D. Roberts‡ A. L. Stewart‡ C. D. Struthers‡ J. E. Robbins NIWA, Private Bag 14901, Wellington 6241 ‡ Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, 6011Wellington ISSN 1176-9440 (print) ISSN 1179-6480 (online) ISBN 978-1-98-859425-5 (print) ISBN 978-1-98-859426-2 (online) 2019 Disclaimer While every effort was made to ensure the information in this publication is accurate, Fisheries New Zealand does not accept any responsibility or liability for error of fact, omission, interpretation or opinion that may be present, nor for the consequences of any decisions based on this information. Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries website at http://www.mpi.govt.nz/news-and-resources/publications/ A higher resolution (larger) PDF of this guide is also available by application to: [email protected] Citation: McMillan, P.J.; Francis, M.P.; James, G.D.; Paul, L.J.; Marriott, P.; Mackay, E.; Wood, B.A.; Stevens, D.W.; Griggs, L.H.; Baird, S.J.; Roberts, C.D.; Stewart, A.L.; Struthers, C.D.; Robbins, J.E. -

Morphology and Phylogenetic Relationships of Fossil Snake Mackerels and Cutlassfishes (Trichiuroidea) from the Eocene (Ypresian) London Clay Formation
MS. HERMIONE BECKETT (Orcid ID : 0000-0003-4475-021X) DR. ZERINA JOHANSON (Orcid ID : 0000-0002-8444-6776) Article type : Original Article Handling Editor: Lionel Cavin Running head: Relationships of London Clay trichiuroids Hermione Becketta,b, Sam Gilesa, Zerina Johansonb and Matt Friedmana,c aDepartment of Earth Sciences, University of Oxford, South Parks Road, Oxford, OX1 3AN, UK bDepartment of Earth Sciences, Natural History Museum, London, SW7 5BD, UK cCurrent address: Museum of Paleontology and Department of Earth and Environmental Sciences, University of Michigan, 1109 Geddes Ave, Ann Arbor, MI 48109-1079, USA *Correspondence to: Hermione Beckett, +44 (0) 1865 272000 [email protected], Department of Earth Sciences, University of Oxford, Oxford, UK, OX1 3AN Short title: Relationships of London Clay trichiuroids Author Manuscript Key words: Trichiuroidea, morphology, London Clay, Trichiuridae, Gempylidae, fossil This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/spp2.1221 This article is protected by copyright. All rights reserved A ‘Gempylids’ (snake mackerels) and trichiurids (cutlassfishes) are pelagic fishes characterised by slender to eel-like bodies, deep-sea predatory ecologies, and large fang-like teeth. Several hypotheses of relationships between these groups have been proposed, but a consensus remains elusive. Fossils attributed to ‘gempylids’ and trichiurids consist almost exclusively of highly compressed body fossils and isolated teeth and otoliths. We use micro-computed tomography to redescribe two three- dimensional crania, historically assigned to †Eutrichiurides winkleri and †Progempylus edwardsi, as well as an isolated braincase (NHMUK PV OR 41318). -

6. Associated Species
57 6. Associated species As mentioned earlier, associated species are those impacted species that are not part of the landed catch. Fisheries for straddling fish stocks, highly migratory fish stocks, and high seas fish stocks, impact other species as a result of: (1) discards, (2) physical contact of fishing gear with organisms (and habitat) that are not caught, and (3) indirect processes. Discards are considered in section 6.1 while physical contact and indirect processes are considered together in section 6.2. 6.1 DISCARDS Much more is known about discards than the other mechanisms through which fisheries impact associated species, although information is still limited. The most recent global information on discards is in an FAO report by Kelleher (2005). It estimates that the rate of discards is about 8 percent for all marine fisheries combined (EEZ and high seas), with large differences by countries, gear types, target species and statistical areas. Shrimp trawling has the highest estimated average discard rate (62.3 percent), but the rates vary widely between fisheries (from 0 to 96 percent). Most shrimp trawling is on stocks confined to the EEZ, although there are some straddling or other high seas stocks of shrimp that are fished. These are likely to be fisheries in relatively deep water for cold-water species, such as the fishery for Pandalus shrimp on the Flemish Cap off Newfoundland and off Labrador in the northwest Atlantic (FAO statistical area 21). The aggregate discard rate for cold/deep-water shrimp fisheries is 39 percent, but where use of bycatch reduction devices (BRDs) is mandated (e.g. -
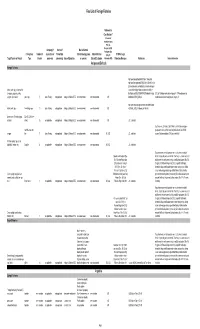
2018 Final LOFF W/ Ref and Detailed Info
Final List of Foreign Fisheries Rationale for Classification ** (Presence of mortality or injury (P/A), Co- Occurrence (C/O), Company (if Source of Marine Mammal Analogous Gear Fishery/Gear Number of aquaculture or Product (for Interactions (by group Marine Mammal (A/G), No RFMO or Legal Target Species or Product Type Vessels processor) processing) Area of Operation or species) Bycatch Estimates Information (N/I)) Protection Measures References Detailed Information Antigua and Barbuda Exempt Fisheries http://www.fao.org/fi/oldsite/FCP/en/ATG/body.htm http://www.fao.org/docrep/006/y5402e/y5402e06.htm,ht tp://www.tradeboss.com/default.cgi/action/viewcompan lobster, rock, spiny, demersal fish ies/searchterm/spiny+lobster/searchtermcondition/1/ , (snappers, groupers, grunts, ftp://ftp.fao.org/fi/DOCUMENT/IPOAS/national/Antigua U.S. LoF Caribbean spiny lobster trap/ pot >197 None documented, surgeonfish), flounder pots, traps 74 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G AndBarbuda/NPOA_IUU.pdf Caribbean mixed species trap/pot are category III http://www.nmfs.noaa.gov/pr/interactions/fisheries/tabl lobster, rock, spiny free diving, loops 19 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented A/G e2/Atlantic_GOM_Caribbean_shellfish.html Queen conch (Strombus gigas), Dive (SCUBA & free molluscs diving) 25 not applicable not applicable Antigua & Barbuda EEZ none documented none documented A/G U.S. trade data Southeastern U.S. Atlantic, Gulf of Mexico, and Caribbean snapper- handline, hook and grouper and other reef fish bottom longline/hook-and-line/ >5,000 snapper line 71 Lewis Fishing not applicable Antigua & Barbuda EEZ none documented none documented N/I, A/G U.S. -

Fish Bulletin 161. California Marine Fish Landings for 1972 and Designated Common Names of Certain Marine Organisms of California
UC San Diego Fish Bulletin Title Fish Bulletin 161. California Marine Fish Landings For 1972 and Designated Common Names of Certain Marine Organisms of California Permalink https://escholarship.org/uc/item/93g734v0 Authors Pinkas, Leo Gates, Doyle E Frey, Herbert W Publication Date 1974 eScholarship.org Powered by the California Digital Library University of California STATE OF CALIFORNIA THE RESOURCES AGENCY OF CALIFORNIA DEPARTMENT OF FISH AND GAME FISH BULLETIN 161 California Marine Fish Landings For 1972 and Designated Common Names of Certain Marine Organisms of California By Leo Pinkas Marine Resources Region and By Doyle E. Gates and Herbert W. Frey > Marine Resources Region 1974 1 Figure 1. Geographical areas used to summarize California Fisheries statistics. 2 3 1. CALIFORNIA MARINE FISH LANDINGS FOR 1972 LEO PINKAS Marine Resources Region 1.1. INTRODUCTION The protection, propagation, and wise utilization of California's living marine resources (established as common property by statute, Section 1600, Fish and Game Code) is dependent upon the welding of biological, environment- al, economic, and sociological factors. Fundamental to each of these factors, as well as the entire management pro- cess, are harvest records. The California Department of Fish and Game began gathering commercial fisheries land- ing data in 1916. Commercial fish catches were first published in 1929 for the years 1926 and 1927. This report, the 32nd in the landing series, is for the calendar year 1972. It summarizes commercial fishing activities in marine as well as fresh waters and includes the catches of the sportfishing partyboat fleet. Preliminary landing data are published annually in the circular series which also enumerates certain fishery products produced from the catch. -

The Increasing Importance of Marine Recreational Fishing in the US
Fisheries Research 108 (2011) 268–276 Contents lists available at ScienceDirect Fisheries Research journal homepage: www.elsevier.com/locate/fishres The increasing importance of marine recreational fishing in the US: Challenges for management Thomas F. Ihde ∗, Michael J. Wilberg, David A. Loewensteiner, David H. Secor, Thomas J. Miller University of Maryland Center for Environmental Science Chesapeake Biological Laboratory, Solomons, MD 20688, USA article info abstract Article history: Harvests from recreational fishing are increasingly as important as commercial harvest to populations of Received 14 May 2010 popularly fished marine recreational species. However, it has yet to be determined whether the increasing Received in revised form 7 December 2010 importance of recreational fishing is a general trend of marine fisheries in the US or whether such a trend Accepted 11 December 2010 is limited to only those species recognized as popular recreational fishes. 71% of marine species in the US have experienced an increase in the proportion of total harvest from the recreational sector during the Keywords: time harvest data are available for both sectors. Species demonstrating an increase in the proportion of Fishery management goals harvests by the recreational sector included those generally regarded as commercial, bait, and bycatch Harvest policy Life history analysis species, as well as those considered recreational species. Marine species categorized as overfished could Marine fisheries management not be predicted from either fishery characteristics or life history characteristics in a PCA analysis of Recreational fisheries available data for fished species in the US. Consequently, there appears to be little to predict vulnerability of populations to fishing efforts save that all fished species can be made vulnerable to overexploitation.