Combined Remote LIBS and Raman Spectroscopy of Minerals Using a Single Laser Source
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
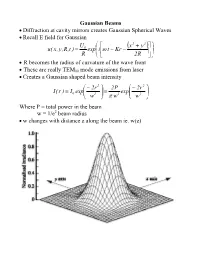
Gaussian Beams • Diffraction at Cavity Mirrors Creates Gaussian Spherical
Gaussian Beams • Diffraction at cavity mirrors creates Gaussian Spherical Waves • Recall E field for Gaussian U ⎛ ⎡ x2 + y2 ⎤⎞ 0 ⎜ ( ) ⎟ u( x,y,R,t ) = exp⎜i⎢ω t − Kr − ⎥⎟ R ⎝ ⎣ 2R ⎦⎠ • R becomes the radius of curvature of the wave front • These are really TEM00 mode emissions from laser • Creates a Gaussian shaped beam intensity ⎛ − 2r 2 ⎞ 2P ⎛ − 2r 2 ⎞ I( r ) I exp⎜ ⎟ exp⎜ ⎟ = 0 ⎜ 2 ⎟ = 2 ⎜ 2 ⎟ ⎝ w ⎠ π w ⎝ w ⎠ Where P = total power in the beam w = 1/e2 beam radius • w changes with distance z along the beam ie. w(z) Measurements of Spotsize • For Gaussian beam important factor is the “spotsize” • Beam spotsize is measured in 3 possible ways • 1/e radius of beam • 1/e2 radius = w(z) of the radiance (light intensity) most common laser specification value 13% of peak power point point where emag field down by 1/e • Full Width Half Maximum (FWHM) point where the laser power falls to half its initial value good for many interactions with materials • useful relationship FWHM = 1.665r1 e FWHM = 1.177w = 1.177r 1 e2 w = r 1 = 0.849 FWHM e2 Gaussian Beam Changes with Distance • The Gaussian beam radius of curvature with distance 2 ⎡ ⎛π w2 ⎞ ⎤ R( z ) = z⎢1 + ⎜ 0 ⎟ ⎥ ⎜ λz ⎟ ⎣⎢ ⎝ ⎠ ⎦⎥ • Gaussian spot size with distance 1 2 2 ⎡ ⎛ λ z ⎞ ⎤ w( z ) = w ⎢1 + ⎜ ⎟ ⎥ 0 ⎜π w2 ⎟ ⎣⎢ ⎝ 0 ⎠ ⎦⎥ • Note: for lens systems lens diameter must be 3w0.= 99% of power • Note: some books define w0 as the full width rather than half width • As z becomes large relative to the beam asymptotically approaches ⎛ λ z ⎞ λ z w(z) ≈ w ⎜ ⎟ = 0 ⎜ 2 ⎟ ⎝π w0 ⎠ π w0 • Asymptotically light -

Imaging with Second-Harmonic Generation Nanoparticles
1 Imaging with Second-Harmonic Generation Nanoparticles Thesis by Chia-Lung Hsieh In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy California Institute of Technology Pasadena, California 2011 (Defended March 16, 2011) ii © 2011 Chia-Lung Hsieh All Rights Reserved iii Publications contained within this thesis: 1. C. L. Hsieh, R. Grange, Y. Pu, and D. Psaltis, "Three-dimensional harmonic holographic microcopy using nanoparticles as probes for cell imaging," Opt. Express 17, 2880–2891 (2009). 2. C. L. Hsieh, R. Grange, Y. Pu, and D. Psaltis, "Bioconjugation of barium titanate nanocrystals with immunoglobulin G antibody for second harmonic radiation imaging probes," Biomaterials 31, 2272–2277 (2010). 3. C. L. Hsieh, Y. Pu, R. Grange, and D. Psaltis, "Second harmonic generation from nanocrystals under linearly and circularly polarized excitations," Opt. Express 18, 11917–11932 (2010). 4. C. L. Hsieh, Y. Pu, R. Grange, and D. Psaltis, "Digital phase conjugation of second harmonic radiation emitted by nanoparticles in turbid media," Opt. Express 18, 12283–12290 (2010). 5. C. L. Hsieh, Y. Pu, R. Grange, G. Laporte, and D. Psaltis, "Imaging through turbid layers by scanning the phase conjugated second harmonic radiation from a nanoparticle," Opt. Express 18, 20723–20731 (2010). iv Acknowledgements During my five-year Ph.D. studies, I have thought a lot about science and life, but I have never thought of the moment of writing the acknowledgements of my thesis. At this moment, after finishing writing six chapters of my thesis, I realize the acknowledgment is probably one of the most difficult parts for me to complete. -

OPTIX Module 1 – Intermediate Introduction to Optics and Optical Elements
OPTIX Module 1 – Intermediate Introduction to optics and optical elements Michaela Kleinert 1 Objectives: In this module you will learn about • the proper use and handling of research-grade optics equipment; • how to use mirrors to align laser beams; • various lenses and their applications; • diffraction gratings and their use in spectrometers; • polarization of light. Use this manual as you work through the module to keep track of your notes and thoughts. In addition, you will have to add a few printouts or add additional sheets of paper containing data tables, sketches, or additional notes. You will not write a separate lab report after this module, because we want to give you enough time to thoroughly familiarize yourself and play with the equipment, but you will be graded on how well you complete this manual. 2 Tests and assessment: In preparation for this module, read through the whole manual and answer the questions that are marked with a *. You should also watch the VIDEOs that are posted on our website (www.willamette.edu/cla/physics/info/NSF-OPTIX). They are meant to accompany this manual and will show you some critical steps of the module. When you come to lab, be prepared to discuss your answers to these questions with your classmates and your instructor. You will also take a short test (“Laser Safety Test”) before you begin working on this module to ensure that you have watched, read, and understood the Laser Safety Material. Lastly, in order to assess the success of this module, you will take a short assessment test before you start (“pre-assessment”), and another one after you have successfully completed this module (“post-assessment”). -

Resolution-And Throughput-Enhanced Spectroscopy Using High-Throughput
Resolution- and throughput-enhanced spectroscopy using high-throughput computational slit Farnoud Kazemzadeh1;∗, and Alexander Wong1 1 Department of Systems Design Engineering, University of Waterloo, Waterloo, Ontario, Canada, N2L 3G1 ∗ E-mail: [email protected] arXiv:1606.09072v2 [physics.optics] 10 Sep 2016 1 There exists a fundamental tradeoff between spectral resolution and the efficiency or throughput for all optical spectrometers. The primary factors affecting the spectral resolution and throughput of an optical spectrometer are the size of the entrance aper- ture and the optical power of the focusing element. Thus far collective optimization of the above mentioned has proven difficult. Here, we introduce the concept of high- throughput computational slits (HTCS), a numerical technique for improving both the effective spectral resolution and efficiency of a spectrometer. The proposed HTCS ap- proach was experimentally validated using an optical spectrometer configured with a 200 µm entrance aperture, test, and a 50 µm entrance aperture, control, demonstrating improvements in spectral resolution of the spectrum by ∼50% over the control spectral resolution and improvements in efficiency of > 2 times over the efficiency of the largest entrance aperture used in the study while producing highly accurate spectra. The spectral resolution and efficiency of an optical spectrometer, which affects the ability to distinguish closely spaced spectral features which are controlled by a number of different factors such as the size and shape of the entrance aperture, the optical characteristics of the dispersive element, the optical characteristics of the collimating optics the focusing optics, and the size and shape of the detector’s pixels. In particular, the size of the entrance aperture (e.g., width of slit used as entrance aperture) and the optical power of the focusing element are the primary factors affecting the tradeoff between spectral resolution and efficiency or throughput of a spectrometer. -

Femtosecond Laser Optics Combat Pulse Dispersion, Color Errors, and Reflections
ULTRAFAST LASER OPTICS Femtosecond laser optics combat pulse dispersion, color errors, and reflections GÜNTER TOESKO and CHRISTIAN DEHNERT Along with the rapid development for improved outcomes Dispersion pace of ultrafast lasers, ultrafast in ophthalmic (Lasik sur- Material dispersion in ultrafast laser op- laser optics must overcome the gery) and biological ap- tics leads to temporal broadening of the plications, the nonlinear laser pulse by introducing a frequency-de- unusually amplified obstacles of attributes of ultrafast la- pendent delay of the different spectral dispersion, color aberrations, and ghost sers are also used to mod- components of the pulse. The higher the reflections through careful design and ify the index of refraction refractive index of a material, the higher manufacture. and distribute the laser the dispersion. In addition, the dispersion energy along the beam effect is greater for shorter wavelengths. The pace of the development of ultra- axis to maintain a near-constant beam For example, a 400-fs-long pulse with a fast lasers—also called ultrashort pulse radius over many Rayleigh lengths, en- central wavelength of 355 nm suffers a (USP) lasers—has been extremely rapid. abling a very large depth of field. temporal broadening of approximately Picosecond and femtosecond laser sys- As a result, USP lasers are well suited to 0.3 fs while traveling through a 20-mm- tems are efficient tools in many indus- contour cutting (filament cutting) of un- thick fused-silica window. trial and scientific applications, offer- cured, chemically hardened glass (cover In long-pulsewidth laser beams, the ing useful nonlinear effects and “cold glass of smartphones) and sapphire, re- wavelength bandwidth is very narrow ablation” for a reduced heat-affected sulting in very high-quality edges and very and typically no compensation is re- zone (HAZ) that reduces or even elim- little material removal. -

3D Atomic Display
3D Atomic Vapor Display Design Description Document UNIVERSITY OF ROCHESTER Design Description Document 3D Atomic Vapor Display Amy Entin, Alexander Rainville, Yucheng Wang, Lindsey Willstatter Customer: Curtis Broadbent Engineers: Amy Entin, Alexander Rainville, Yucheng Wang, Lindsey Willstatter Advisor committee: Curtis Broadbent Document Number: 04 Date: Revision Level: 04/26/16 E Authentication Block This is a computer generated document and the electronic master is the official revision. This paper copy is authenticated for the following purpose only: 01Rev E| 1 3D Atomic Vapor Display Design Description Document Contents Project Summary System Block Diagram Base Layout Designs 3D Drawings Optical Design Lens System Flood Beam Design Optical Transmission Mechanical Housing for Optics Beam Deviation in Sphere Test Plan / Validation Risk Assessment Transition Plans Appendix I: Product Requirement Document Appendix II: Bill of Materials 01Rev E| 2 3D Atomic Vapor Display Design Description Document Project summary: The system consists of a device to scan two laser beams to an intersection in a sphere. Each scanner system consists of a laser, collimation optics, two beam steering mirrors, 2D galvo scanners and final mirrors. In addition, one of these scanners is joined by a second laser, and the system is illuminated from below using a fourth laser and a convex mirror. The system must fit on a 18” circular base. System Block Diagram 1. Two laser systems, as ordered by the customer. 2. Collimating optics. 3. 2D Galvo scanners for steering -

Characterization of Yb:YAG Active Slab Media Based on a Layered Structure with Different Doping
Characterization of Yb:YAG active slab media based on a layered structure with different doping A.Lapucci*a, M.Ciofini a, L.Espositob, P.Ferrarac,L.A.Gizzic, J.Hostašab,e, L.Labatec, A.Pirrid, G.Tocid, M.Vanninid a C.N.R. - National Research Council, Istituto Nazionale di Ottica (CNR-INO), Largo Enrico Fermi 6, I-50125 Firenze, Italy; b C.N.R. - National Research Council, Institute of Science and Technology for Ceramics (CNR-ISTEC), Via Granarolo 64, 48018 Faenza, Italy; c C.N.R. - National Research Council, Istituto Nazionale di Ottica (CNR-INO), Via G. Moruzzi, 1 - I-56124 Pisa, Italy; d C.N.R. - National Research Council, Institute of Applied Physics "Carrara" (CNR-IFAC), Via Madonna del Piano 10, I-50019 Sesto Fiorentino (FI) Italy; e Department of Glass and Ceramics, ICT Prague, Technická 5, 166 28 Prague, Czech Republic ABSTRACT Slabs with non-uniform doping distribution are studied with the aim of reducing thermal deformations in high-energy high-average-power Yb:YAG slab systems. We present a numerical analysis based on Finite Element Mesh (FEM) methods suitable to model non-uniform devices. The thermal variation of the refractive index, the end-faces deformations and the photo-elastic effect have been calculated in order to estimate the total thermal-lens effect. The stress distributions are also obtained. Some results of this numerical approach are compared to experimental thermal lens measurements in a simple geometry for both uniform and structured samples, in order to validate the numerical procedures. Finally we compare numerical simulations for different single- or double-sided pumping and cooling geometries. -

Lenses, Magnification & Beam Expanders
Dept. Electrical Engin. & 1 Lecture 2 – Lenses Computing Systems 2 – Lenses, Magnification & Beam Expanders EECS 6048 – Optics for Engineers © Instructor – Prof. Jason Heikenfeld Dept. Electrical Engin. & 2 Lecture 2 – Lenses Computing Systems 2 – Lenses, Magnification & Beam Expanders EECS 6048 – Optics for Engineers © Instructor – Prof. Jason Heikenfeld Dept. Electrical Engin. & 3 Note for the quiz! Computing Systems Notice for quiz: starting this week, you will have had used several of the equations we learned in lecture in the lab and lab homework, and therefore the quiz will also start to incorporate calculation style problems from the previous weeks lab. I don’t expect you to memorize the equations, you will always be able to look them up anyway… Therefore each week you may bring to the quiz, 1/3rd of a sheet of paper with anything you want on it, anything! You can keep adding to it, for example, such that 3 weeks from now it is a full sheet, and then you start a 2nd sheet, etc… EECS 6048 – Optics for Engineers © Instructor – Prof. Jason Heikenfeld Dept. Electrical Engin. & 4 Today… Computing Systems ! Today, we will only need to consider ray optics… Credit: Fund. Photonics – Fig. 2.3-1 Credit: Fund. Photonics – Fig. 1.0-1 ! Topics: (1) derive the basic lens formula (2) positive and negative lenses, and imaging planes (3) multiple lenses in series (beam expanders, telescopes) (4) advanced stuff (microscopes, numerical aperture, variable focus lenses) Figures today are mainly from CH1 of Fund. of Photonics or wiki. EECS 6048 – Optics for Engineers © Instructor – Prof. Jason Heikenfeld Dept. -

ABSTRACT BOKHART, MARK THOMAS. Development And
ABSTRACT BOKHART, MARK THOMAS. Development and Application of Matrix-Assisted Laser Desorption Electrospray Ionization Mass Spectrometry Imaging for Drug Distribution Studies. (Under the direction of Dr. David C. Muddiman.) Infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) is a hybrid ionization technique combining resonant laser ablation and electrospray ionization. Analysis in a spatially resolved manner permits mass spectrometry imaging (MSI) analyses to be performed by displaying ion intensities in a 2-dimension array. IR-MALDESI has several unique advantages over conventional MSI techniques, for example matrix-assisted laser desorption/ionization (MALDI), such as operation at ambient conditions and the use of a biologically-compatible ice matrix. Additionally, the mid-infrared (IR) laser used for ablation has much greater ablation depth compared to ultraviolet lasers used in MALDI, usually completely ablating the sampled material at each rastered position. This attribute provides quantitative sampling of material at each position, giving high quality MSI data. The IR-MALDESI MSI source was optimized through a systematic investigation of factors effecting desorption of analytes including IR laser wavelength, geometric considerations and the inclusion of ice as a matrix. Additional optimization was performed by investigating trends in analyte response as the mass spectrometer C-trap injection time was varied. The shorter C-trap injection times lead to increases in ion abundance and identification of more species in untargeted analyses. A quantitative mass spectrometry imaging method was developed for use with IR- MALDESI, where a normalization compound is uniformly incorporated beneath the tissue to be quantified along with a spatial calibration curve incorporated on top of the same tissue. -

UV Optics Brochure
Edmund Optics® BROCHURE EDMUND OPTICS® ULTRAVIOLET OPTICS ® COPYRIGHT 2018 EDMUND OPTICS, INC. ALL RIGHTS RESERVED 1/18 RIGHTS RESERVED ALL 2018 EDMUND OPTICS, INC. ® COPYRIGHT TECHNICAL RESOURCES | PRODUCTS | CASE STUDY Contact us for a Stock or Custom Quote Today! USA: +1-856-547-3488 | EUROPE: +44 (0) 1904 788600 ASIA: +65 6273 6644 | JAPAN: +81-3-3944-6210 www.edmundoptics.com/uv-optics Tech Support & ORDERING MADE EASY Edmund Optics® (EO) is a premier supplier of UV optics including mirrors, lenses, filters, and beam expanders. EO offers free engineering and technical support, along with an extensive technical library of online videos, application notes, and calculators. Additional product documentation including over 37,000 data sheets, Zemax files, 3D models, and filter curves are also available. Why Edmund Optics®? Quality • ISO 9001 Certified and MIL-SPEC quality systems • Wide range of metrology including interferometers, cavity ring-down (CRD) spectrometers, Shack-Hartmann wavefront sensors, profilometers, and coordinate measurement machines (CMMs) Capabilities and Service • Optics manufacturing in USA, Europe, and Asia • Custom design services emphasizing manufacturability • Rapid turnaround of modification services TEXT PHONE & ONLINE CHAT Text us from your mobile device to speak with one 800.363.1992 or 856.547.3488 of our team members. Message & data rates may apply. Mon - Fri, 8AM - 8PM EST | Sat, 10AM - 1PM EST [email protected] | www.edmundoptics.com/contact Technical Support: 1.856.547.3488 | Mon - Fri, 8AM - 8PM EST 24-HOUR ENGINEERING SUPPORT 800.363.1992 or 856.547.3488 Sales Support: Sun, 8PM - Fri, 8PM EST | [email protected] 1.856.559.7889 | Mon - Fri, 8AM - 8PM EST Sat, 10AM - 1PM EST TECHNICAL EXPERTS AT YOUR SERVICE The optics EO makes enable the future and I love developing technology and product that inevitably helps customers achieve their goals. -

BACHELOR THESIS Design and Construction of an Infrared Laser
BACHELOR THESIS Deggendorf Institute of Technology University of Applied Science Faculty of Applied Natural Sciences and Industrial Engineering Course of Engineering Physics Design and Construction of an Infrared Laser Transmitter for a Compact Satellite Laser Ranging System (Design und Aufbau eines Infrarot Laser Transmitters für ein Kompaktes Satellite Laser Ranging System) Bachelor Thesis in Fulfilment of the Requirements for the Degree Bachelor of Engineering (B.Eng.) Submitted by: Pia Lützen, Deggendorf 570309 Supervisor: Prof. Dr. Josef Kölbl Deggendorf, 06.03.2019 Erklärung Name d. Studierenden: Pia Lützen Name d. Betreuers: Prof. Dr. Josef Kölbl Thema der Bachelorarbeit: Design und Aufbau eines Infrarot Lasertransmitters für ein kompaktes Satellite Laser Ranging System 1. Ich erkläre hiermit, dass ich die Bachelorarbeit selbstständig verfasst, noch nicht anderweitig für Prüfungszwecke vorgelegt, keine anderen als die angegeben Quellen oder Hilfsmittel benutzt sowie wörtliche und sinngemäße Zitate als solche gekennzeichnet habe. Deggendorf, den __________ Unterschrift d. Studierenden: ________________________ 2. Ich bin damit einverstanden, dass die von mir angefertigte Bachelorarbeit über die Bibliothek der Hochschule einer breiteren Öffentlichkeit zugänglich gemacht wird. Ja Nein Ich erkläre und stehe dafür ein, dass ich alleiniger Inhaber aller Rechte an der Bachelorarbeit, einschließlich des Verfügungsrechts über Vorlagen an beigefügten Abbildungen, Plänen o.ä., bin und durch deren öffentliche Zugänglichmachung weder Rechte und Ansprüche Dritter noch gesetzliche Bestimmungen verletzt werden. Deggendorf, den __________ Unterschrift d. Studierenden: ________________________ Bei Einverständnis des Verfassers mit einer Zugänglichmachung der Bachelorarbeit vom Betreuer auszufüllen: 3. Eine Aufnahme eines Exemplars der Bachelorarbeit in den Bestand der Bibliothek und die Ausleihe des Exemplars wird befürwortet nicht befürwortet Deggendorf, den __________ Unterschrift d. -

2012 Second Harmonic Generation Imaging of the Deep Shade Plant
Journal of Microscopy, Vol. 248, Pt 3 2012, pp. 234–244 doi: 10.1111/j.1365-2818.2012.03668.x Received 4 April 2012; accepted 28 August 2012 Second harmonic generation imaging of the deep shade plant Selaginella erythropus using multifunctional two-photon laser scanning microscopy A.H. RESHAK∗,† & C.-R. SHEUE‡ ∗School of Complex Systems, FFPW, CENAKVA, University of South Bohemia in CB, Nove Hrady 37333, Czech Republic †School of Material Engineering, Malaysia University of Perlis, P.O Box 77, d/a Pejabat Pos Besar, 01007 Kangar, Perlis, Malaysia ‡Department of Life Sciences, National Chung Hsing University, 250, Kuo Kuang Rd, Taichung 402, Taiwan Key words. Chloroplast, multifunctional two-photon laser scanning microscopy (MF-2PLSM), second harmonic generation (SHG), Selaginella erythropus, two-photon excitation fluorescence (TPEF). Summary SHG is known to leave no energy deposition on the interacting matter because of the SHG virtual energy conservation Background: Multifunctional two-photon laser scanning characteristic. microscopy provides attractive advantages over conventional two-photon laser scanning microscopy. For the first time, simultaneous measurement of the second harmonic Introduction generation (SHG) signals in the forward and backward directions and two photon excitation fluorescence were Nonlinear optical effects, such as two-photon (Denk et al., achieved from the deep shade plant Selaginella erythropus. 1990) and three-photon (Wokosin et al., 1996; Maiti et al., Results: These measurements show that the S. erythropus 1997; Schrader et al., 1997; Tuer et al., 2008) fluorescence, leaves produce high SHG signals in both directions and significantly improve depth resolution and reduce the the SHG signals strongly depend on the laser’s status of background noise.