Imaging with Second-Harmonic Generation Nanoparticles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Second Harmonic Imaging Microscopy
170 Microsc Microanal 9(Suppl 2), 2003 DOI: 10.1017/S143192760344066X Copyright 2003 Microscopy Society of America Second Harmonic Imaging Microscopy Leslie M. Loew,* Andrew C. Millard,* Paul J. Campagnola,* William A. Mohler,* and Aaron Lewis‡ * Center for Biomedical Imaging Technology, University of Connecticut Health Center, Farmington, CT 06030-1507 USA ‡ Division of Applied Physics, Hebrew University of Jerusalem, Jerusalem 91904, Israel Second Harmonic Generation (SHG) has been developed in our laboratories as a high- resolution non-linear optical imaging microscopy (“SHIM”) for cellular membranes and intact tissues. SHG is a non-linear process that produces a frequency doubling of the intense laser field impinging on a material with a high second order susceptibility. It shares many of the advantageous features for microscopy of another more established non-linear optical technique: two-photon excited fluorescence (TPEF). Both are capable of optical sectioning to produce 3D images of thick specimens and both result in less photodamage to living tissue than confocal microscopy. SHG is complementary to TPEF in that it uses a different contrast mechanism and is most easily detected in the transmitted light optical path. It also does not arise via photon emission from molecular excited states, as do both 1- and 2-photon excited fluorescence. SHG of intrinsic highly ordered biological structures such as collagen has been known for some time but only recently has the full potential of high resolution 3D SHIM been demonstrated on live cells and tissues. For example, Figure 1 shows SHIM from microtubules in a living organism, C. elegans. The images were obtained from a transgenic nematode that expresses a ß-tubulin-green fluorescent protein fusion and Figure 1 also shows the TPEF image from this molecule for comparison. -

Multiphoton Microscopy
Living up to Life Multiphoton Microscopy 1 Jablonski Diagram: Living up to Life Nonlinear Optical Microscopy F.- Helmchen, W. Denk, Deep tissue two-photon microscopy, Nat. Methods 2, 932-940 2 Typical Samples – Living up to Life Small Dimensions & Highly Scattering • Somata 10-30 µm • Dendrites 1-5 µm • Spines ~0.5 µm • Axons 1-2 µm ls ~ 50-100 µm (@ 630 nm) ls ~ 200 µm (@ 800 nm) T. Nevian Institute of Physiology University of Bern, Switzerland F.- Helmchen, W. Denk Deep tissue two-photon microscopy. Nat. Methods 2, 932-940 3 Why Multiphoton microscopy? Living up to Life • Today main challenge: To go deeper into samples for improved studies of cells, organs or tissues, live animals Less photodamage, i.e. less bleaching and phototoxicity • Why is it possible? Due to the reduced absorption and scattering of the excitation light 4 The depth limit Living up to Life • Achievable depth: ~ 300 – 600 µm • Maximum imaging depth depends on: – Available laser power – Scattering mean-free-path – Tissue properties • Density properties • Microvasculature organization • Cell-body arrangement • Collagen / myelin content – Specimen age – Collection efficiency Acute mouse brain sections containing YFP neurons,maximum projection, Z stack: 233 m Courtesy: Dr Feng Zhang, Deisseroth laboratory, Stanford University, USA Page 5 What is Two‐Photon Microscopy? Living up to Life A 3-dimensional imaging technique in which 2 photons are used to excite fluorescence emission exciting photon emitted photon S1 Simultaneous absorption of 2 longer wavelength photons to -

Single-Pass Laser Frequency Conversion to 780.2 Nm and 852.3 Nm Based on Ppmgo:LN Bulk Crystals and Diode-Laser-Seeded Fiber Amplifiers
applied sciences Article Single-Pass Laser Frequency Conversion to 780.2 nm and 852.3 nm Based on PPMgO:LN Bulk Crystals and Diode-Laser-Seeded Fiber Amplifiers Kong Zhang 1, Jun He 1,2 and Junmin Wang 1,2,* 1 State Key Laboratory of Quantum Optics and Quantum Optics Devices, and Institute of Opto-Electronics, Shanxi University, Taiyuan 030006, China; [email protected] (K.Z.); [email protected] (J.H.) 2 Collaborative Innovation Center of Extreme Optics of the Ministry of Education and Shanxi Province, Shanxi University, Taiyuan 030006, China * Correspondence: [email protected] Received: 16 October 2019; Accepted: 15 November 2019; Published: 17 November 2019 Abstract: We report the preparation of a 780.2 nm and 852.3 nm laser device based on single-pass periodically poled magnesium-oxide-doped lithium niobate (PPMgO:LN) bulk crystals and diode-laser-seeded fiber amplifiers. First, a single-frequency continuously tunable 780.2 nm laser of more than 600 mW from second-harmonic generation (SHG) by a 1560.5 nm laser can be achieved. Then, a 250 mW light at 852.3 nm is generated and achieves an overall conversion efficiency of 4.1% from sum-frequency generation (SFG) by mixing the 1560.5 nm and 1878.0 nm lasers. The continuously tunable range of 780.2 nm and 852.3 nm are at least 6.8 GHz and 9.2 GHz. By employing this laser system, we can conveniently perform laser cooling, trapping and manipulating both rubidium (Rb) and cesium (Cs) atoms simultaneously. This system has promising applications in a cold atoms Rb-Cs two-component interferemeter and in the formation of the RbCs dimer by the photoassociation of cold Rb and Cs atoms confined in a magneto-optical trap. -

Second Harmonic Generation in Nonlinear Optical Crystal
Second Harmonic Generation in Nonlinear Optical Crystal Diana Jeong 1. Introduction In traditional electromagnetism textbooks, polarization in the dielectric material is linearly proportional to the applied electric field. However since in 1960, when the coherent high intensity light source became available, people realized that the linearity is only an approximation. Instead, the polarization can be expanded in terms of applied electric field. (Component - wise expansion) (1) (1) (2) (3) Pk = ε 0 (χ ik Ei + χ ijk Ei E j + χ ijkl Ei E j Ek +L) Other quantities like refractive index (n) can be expanded in terms of electric field as well. And the non linear terms like second (E^2) or third (E^3) order terms become important. In this project, the optical nonlinearity is present in both the source of the laser-mode-locked laser- and the sample. Second Harmonic Generation (SHG) is a coherent optical process of radiation of dipoles in the material, dependent on the second term of the expansion of polarization. The dipoles are oscillated with the applied electric field of frequency w, and it radiates electric field of 2w as well as 1w. So the near infrared input light comes out as near UV light. In centrosymmetric materials, SHG cannot be demonstrated, because of the inversion symmetries in polarization and electric field. The only odd terms survive, thus the second order harmonics are not present. SHG can be useful in imaging biological materials. For example, the collagen fibers and peripheral nerves are good SHG generating materials. Since the SHG is a coherent process it, the molecules, or the dipoles are not excited in terms of the energy levels. -

Adaptive Optics in Microscopy
Downloaded from http://rsta.royalsocietypublishing.org/ on February 3, 2015 Phil. Trans. R. Soc. A (2007) 365, 2829–2843 doi:10.1098/rsta.2007.0013 Published online 13 September 2007 Adaptive optics in microscopy BY MARTIN J. BOOTH* Department of Engineering Science, University of Oxford, Parks Road, Oxford OX1 3PJ, UK The imaging properties of optical microscopes are often compromised by aberrations that reduce image resolution and contrast. Adaptive optics technology has been employed in various systems to correct these aberrations and restore performance. This has required various departures from the traditional adaptive optics schemes that are used in astronomy. This review discusses the sources of aberrations, their effects and their correction with adaptive optics, particularly in confocal and two-photon microscopes. Different methods of wavefront sensing, indirect aberration measurement and aberration correction devices are discussed. Applications of adaptive optics in the related areas of optical data storage, optical tweezers and micro/nanofabrication are also reviewed. Keywords: adaptive optics; aberrations; confocal microscopy; multiphoton microscopy; optical data storage; optical tweezers 1. Introduction Optical microscopes are essential tools in many scientific fields. In the life sciences, they are widely used for the visualization of cellular structures and sub- cellular processes. Confocal and multiphoton microscopes are particularly important in this respect as they produce three-dimensional images of volumetric objects. However, the resolution of these microscopes is often adversely affected by the optical properties of the specimen itself. Spatial variations in the refractive index of the specimen introduce optical aberrations that compromise image quality. This is a particular problem when imaging deep into thick biological specimens. -
Fv1000 Fluoview
Confocal Laser Scanning Biological Microscope FV1000 FLUOVIEW FLUOVIEW—Always Evolving FLUOVIEW–—From Olympus is Open FLUOVIEW—More Advanced than Ever The Olympus FLUOVIEW FV1000 confocal laser scanning microscope delivers efficient and reliable performance together with the high resolution required for multi-dimensional observation of cell and tissue morphology, and precise molecular localization. The FV1000 incorporates the industry’s first dedicated laser light stimulation scanner to achieve simultaneous targeted laser stimulation and imaging for real-time visualization of rapid cell responses. The FV1000 also measures diffusion coefficients of intracellular molecules, quantifying molecular kinetics. Quite simply, the FLUOVIEW FV1000 represents a new plateau, bringing “imaging to analysis.” Olympus continues to drive forward the development of FLUOVIEW microscopes, using input from researchers to meet their evolving demands and bringing “imaging to analysis.” Quality Performance with Innovative Design FV10i 1 Imaging to Analysis ing up New Worlds From Imaging to Analysis FV1000 Advanced Deeper Imaging with High Resolution FV1000MPE 2 Advanced FLUOVIEW Systems Enhance the Power of Your Research Superb Optical Systems Set the Standard for Accuracy and Sensitivity. Two types of detectors deliver enhanced accuracy and sensitivity, and are paired with a new objective with low chromatic aberration, to deliver even better precision for colocalization analysis. These optical advances boost the overall system capabilities and raise performance to a new level. Imaging, Stimulation and Measurement— Advanced Analytical Methods for Quantification. Now equipped to measure the diffusion coefficients of intracellular molecules, for quantification of the dynamic interactions of molecules inside live cell. FLUOVIEW opens up new worlds of measurement. Evolving Systems Meet the Demands of Your Application. -

Nonlinear Optical Characterization of 2D Materials
nanomaterials Review Nonlinear Optical Characterization of 2D Materials Linlin Zhou y, Huange Fu y, Ting Lv y, Chengbo Wang y, Hui Gao, Daqian Li, Leimin Deng and Wei Xiong * Wuhan National Laboratory for Optoelectronics, School of Optical and Electronic Information, Huazhong University of Science and Technology, Wuhan 430074, China; [email protected] (L.Z.); [email protected] (H.F.); [email protected] (T.L.); [email protected] (C.W.); [email protected] (H.G.); [email protected] (D.L.); [email protected] (L.D.) * Correspondence: [email protected] These authors contributed equally to this work. y Received: 7 October 2020; Accepted: 30 October 2020; Published: 16 November 2020 Abstract: Characterizing the physical and chemical properties of two-dimensional (2D) materials is of great significance for performance analysis and functional device applications. As a powerful characterization method, nonlinear optics (NLO) spectroscopy has been widely used in the characterization of 2D materials. Here, we summarize the research progress of NLO in 2D materials characterization. First, we introduce the principles of NLO and common detection methods. Second, we introduce the recent research progress on the NLO characterization of several important properties of 2D materials, including the number of layers, crystal orientation, crystal phase, defects, chemical specificity, strain, chemical dynamics, and ultrafast dynamics of excitons and phonons, aiming to provide a comprehensive review on laser-based characterization for exploring 2D material properties. Finally, the future development trends, challenges of advanced equipment construction, and issues of signal modulation are discussed. In particular, we also discuss the machine learning and stimulated Raman scattering (SRS) technologies which are expected to provide promising opportunities for 2D material characterization. -

Efficient Frequency Doubling at 399Nm Arxiv:1401.1623V3 [Physics
Efficient Frequency Doubling at 399 nm Marco Pizzocaro,1, 2, ∗ Davide Calonico,2 Pablo Cancio Pastor,3, 4 Jacopo Catani,3, 4 Giovanni A. Costanzo,1 Filippo Levi,2 and Luca Lorini2 1Politecnico di Torino, Dipartimento di Elettronica e Telecomunicazioni, C.so duca degli Abruzzi 24, 10125 Torino, Italy 2Istituto Nazionale di Ricerca Metrologica (INRIM), Str. delle Cacce 91, 10135 Torino, Italy 3Istituto Nazionale di Ottica (INO-CNR), Via Nello Carrara, 1, 50019 Sesto Fiorentino, Italy 4European Laboratory for Non-Linear Spectroscopy (LENS), Via Nello Carrara, 1, 50019 Sesto Fiorentino, Italy (Dated: May 22, 2014) Abstract This article describes a reliable, high-power, and narrow-linewidth laser source at 399 nm useful for cooling and trapping of ytterbium atoms. A continuous-wave titanium-sapphire laser at 798 nm is frequency doubled using a lithium triborate crystal in an enhancement cavity. Up to 1:0 W of light at 399 nm has been obtained from 1:3 W of infrared light, with an efficiency of 80 %. arXiv:1401.1623v3 [physics.optics] 21 May 2014 ∗ Author to whom correspondence should be addressed. Electronic mail: [email protected] 1 Ytterbium holds interest for several atomic physics experiments because it is easy to cool and trap and has seven stable isotopes. Such experiments include optical frequency standards [1], non-conservation measurement [2], Bose-Einstein condensation [3], degenerate Fermi gases [4,5], bosonic-fermionic systems [6], and quantum information [7]. Ytterbium atoms can be cooled at millikelvin temperature using a magneto-optical trap (MOT) on the 1 1 strong blue transition S0− P1 at 399 nm. -

Optical Sectioning in Fluorescence Microscopy by Confocal and 2
Optical Sectioning in Fluorescence achieved with this methodology, Calcium sparks are microscopic calcium release Downloaded from events inside living muscle cells and their properties are giving new insight into Microscopy by Confocal and how excitation leads to contraction (Cannell et al., 1995; Lopez-Lopez et al,, 2-Photon Molecular Excitation 1995; Gomez et al., 1997). Although the wide field microscope had been applied to calcium imaging since about 1985, calcium sparks had not been observed Techniques previously. This is probably because the presence of fluorescence from outside https://www.cambridge.org/core M.B. Cannell & C.Soeller the focal plane results in a marked loss of in-plane contrast for wide field St. George's Hospital Medical School, microscopy. (Note also that fluo-3 was used as the calcium indicator in these Cranmer Terrace, London SW17 ORE experiments as it has low fluorescence in the absence of calcium which also improves image contrast.) The calcium spark illustrates the high sensitivity of Confocal Microscopy current confocal optical methods - the calcium spark finally occupies about 10 fl Fluorescence microscopy has proved to be an invaluable tool for 14 4 (10" l) and represents calcium binding to only -10 indicator molecules, Until biomedical science since it is possible to visualise small quantities of labeled recently, the laser scanning confocal microscope has been the only instrument materials (such as intracellular ions and proteins) in both fixed and living that could measure fluorescence with a spatial resolution of about 0.4 x 0.4 x 0.8 cells, However, the conventional wide field fluorescence microscope suffers . -
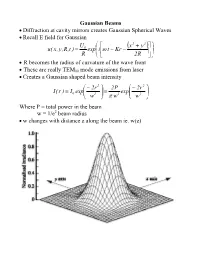
Gaussian Beams • Diffraction at Cavity Mirrors Creates Gaussian Spherical
Gaussian Beams • Diffraction at cavity mirrors creates Gaussian Spherical Waves • Recall E field for Gaussian U ⎛ ⎡ x2 + y2 ⎤⎞ 0 ⎜ ( ) ⎟ u( x,y,R,t ) = exp⎜i⎢ω t − Kr − ⎥⎟ R ⎝ ⎣ 2R ⎦⎠ • R becomes the radius of curvature of the wave front • These are really TEM00 mode emissions from laser • Creates a Gaussian shaped beam intensity ⎛ − 2r 2 ⎞ 2P ⎛ − 2r 2 ⎞ I( r ) I exp⎜ ⎟ exp⎜ ⎟ = 0 ⎜ 2 ⎟ = 2 ⎜ 2 ⎟ ⎝ w ⎠ π w ⎝ w ⎠ Where P = total power in the beam w = 1/e2 beam radius • w changes with distance z along the beam ie. w(z) Measurements of Spotsize • For Gaussian beam important factor is the “spotsize” • Beam spotsize is measured in 3 possible ways • 1/e radius of beam • 1/e2 radius = w(z) of the radiance (light intensity) most common laser specification value 13% of peak power point point where emag field down by 1/e • Full Width Half Maximum (FWHM) point where the laser power falls to half its initial value good for many interactions with materials • useful relationship FWHM = 1.665r1 e FWHM = 1.177w = 1.177r 1 e2 w = r 1 = 0.849 FWHM e2 Gaussian Beam Changes with Distance • The Gaussian beam radius of curvature with distance 2 ⎡ ⎛π w2 ⎞ ⎤ R( z ) = z⎢1 + ⎜ 0 ⎟ ⎥ ⎜ λz ⎟ ⎣⎢ ⎝ ⎠ ⎦⎥ • Gaussian spot size with distance 1 2 2 ⎡ ⎛ λ z ⎞ ⎤ w( z ) = w ⎢1 + ⎜ ⎟ ⎥ 0 ⎜π w2 ⎟ ⎣⎢ ⎝ 0 ⎠ ⎦⎥ • Note: for lens systems lens diameter must be 3w0.= 99% of power • Note: some books define w0 as the full width rather than half width • As z becomes large relative to the beam asymptotically approaches ⎛ λ z ⎞ λ z w(z) ≈ w ⎜ ⎟ = 0 ⎜ 2 ⎟ ⎝π w0 ⎠ π w0 • Asymptotically light -

Two-Photon Excitation Fluorescence Microscopy
P1: FhN/ftt P2: FhN July 10, 2000 11:18 Annual Reviews AR106-15 Annu. Rev. Biomed. Eng. 2000. 02:399–429 Copyright c 2000 by Annual Reviews. All rights reserved TWO-PHOTON EXCITATION FLUORESCENCE MICROSCOPY PeterT.C.So1,ChenY.Dong1, Barry R. Masters2, and Keith M. Berland3 1Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139; e-mail: [email protected] 2Department of Ophthalmology, University of Bern, Bern, Switzerland 3Department of Physics, Emory University, Atlanta, Georgia 30322 Key Words multiphoton, fluorescence spectroscopy, single molecule, functional imaging, tissue imaging ■ Abstract Two-photon fluorescence microscopy is one of the most important re- cent inventions in biological imaging. This technology enables noninvasive study of biological specimens in three dimensions with submicrometer resolution. Two-photon excitation of fluorophores results from the simultaneous absorption of two photons. This excitation process has a number of unique advantages, such as reduced specimen photodamage and enhanced penetration depth. It also produces higher-contrast im- ages and is a novel method to trigger localized photochemical reactions. Two-photon microscopy continues to find an increasing number of applications in biology and medicine. CONTENTS INTRODUCTION ................................................ 400 HISTORICAL REVIEW OF TWO-PHOTON MICROSCOPY TECHNOLOGY ...401 BASIC PRINCIPLES OF TWO-PHOTON MICROSCOPY ..................402 Physical Basis for Two-Photon Excitation ............................ -

Innovations of Wide-Field Optical-Sectioning
Innovations of wide-field optical-sectioning fluorescence microscopy: toward high-speed volumetric bio-imaging with simplicity Thesis by Jiun-Yann Yu In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy California Institute of Technology Pasadena, California 2014 (Defended March 25, 2014) ii c 2014 Jiun-Yann Yu All Rights Reserved iii Acknowledgements Firstly, I would like to thank my thesis advisor, Professor Chin-Lin Guo, for all of his kind advice and generous financial support during these five years. I would also like to thank all of the faculties in my thesis committee: Professor Geoffrey A. Blake, Professor Scott E. Fraser, and Professor Changhuei Yang, for their guidance on my way towards becoming a scientist. I would like to specifically thank Professor Blake, and his graduate student, Dr. Daniel B. Holland, for their endless kindness, enthusiasms and encouragements with our collaborations, without which there would be no more than 10 pages left in this thesis. Dr. Thai Truong of Prof. Fraser's group and Marco A. Allodi of Professor Blake's group are also sincerely acknowledged for contributing to this collaboration. All of the members of Professor Guo's group at Caltech are gratefully acknowledged. I would like to thank our former postdoctoral scholar Dr. Yenyu Chen for generously teaching me all the engineering skills I need, and passing to me his pursuit of wide-field optical-sectioning microscopy. I also thank Dr. Mingxing Ouyang for introducing me the basic concepts of cell biology and showing me the basic techniques of cell-biology experiments. I would like to pay my gratefulness to our administrative assistant, Lilian Porter, not only for her help on administrative procedures, but also for her advice and encouragement on my academic career in the future.