FAQ What Is a Satellite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In-Depth Review of Satellite Imagery / Earth Observation Technology in Official Statistics Prepared by Canada and Mexico
In-depth review of satellite imagery / earth observation technology in official statistics Prepared by Canada and Mexico Julio A. Santaella Conference of European Statisticians 67th plenary session Paris, France June 28, 2019 Earth observation (EO) EO is the gathering of information about planet Earth’s physical, chemical and biological systems. It involves monitoring and assessing the status of, and changes in, the natural and man-made environment Measurements taken by a thermometer, wind gauge, ocean buoy, altimeter or seismograph Photographs and satellite imagery Radar and sonar images Analyses of water or soil samples EO examples EO Processed information such as maps or forecasts Source: Group on Earth Observations (GEO) In-depth review of satellite imagery / earth observation technology in official statistics 2 Introduction Satellite imagery uses have expanded over time Satellite imagery provide generalized data for large areas at relatively low cost: Aligned with NSOs needs to produce more information at lower costs NSOs are starting to consider EO technology as a data collection instrument for purposes beyond agricultural statistics In-depth review of satellite imagery / earth observation technology in official statistics 3 Scope and definition of the review To survey how various types of satellite data and the techniques used to process or analyze them support the GSBPM To improve coordination of statistical activities in the UNECE region, identify gaps or duplication of work, and address emerging issues In-depth review of satellite imagery / earth observation technology in official statistics 4 Overview of recent activities • EO technology has developed progressively, encouraging the identification of new applications of this infrastructure data. -

Detecting, Tracking and Imaging Space Debris
r bulletin 109 — february 2002 Detecting, Tracking and Imaging Space Debris D. Mehrholz, L. Leushacke FGAN Research Institute for High-Frequency Physics and Radar Techniques, Wachtberg, Germany W. Flury, R. Jehn, H. Klinkrad, M. Landgraf European Space Operations Centre (ESOC), Darmstadt, Germany Earth’s space-debris environment tracked, with estimates for the number of Today’s man-made space-debris environment objects larger than 1 cm ranging from 100 000 has been created by the space activities to 200 000. that have taken place since Sputnik’s launch in 1957. There have been more than 4000 The sources of this debris are normal launch rocket launches since then, as well as many operations (Fig. 2), certain operations in space, other related debris-generating occurrences fragmentations as a result of explosions and such as more than 150 in-orbit fragmentation collisions in space, firings of satellite solid- events. rocket motors, material ageing effects, and leaking thermal-control systems. Solid-rocket Among the more than 8700 objects larger than 10 cm in Earth orbits, motors use aluminium as a catalyst (about 15% only about 6% are operational satellites and the remainder is space by mass) and when burning they emit debris. Europe currently has no operational space surveillance aluminium-oxide particles typically 1 to 10 system, but a powerful radar facility for the detection and tracking of microns in size. In addition, centimetre-sized space debris and the imaging of space objects is available in the form objects are formed by metallic aluminium melts, of the 34 m dish radar at the Research Establishment for Applied called ‘slag’. -

Spacecraft Network Operations Demonstration Using
Nodes Spacecraft Network Operations Demonstration Using Multiple Spacecraft in an Autonomously Configured Space Network Allowing Crosslink Communications and Multipoint Scientific Measurements Nodes is a technology demonstration mission that was launched to the International Space Station on December 6, 2015. The two Nodes satellites subsequently deployed from the Station on May 16, 2016 to demonstrate new network capabilities critical to the operation of swarms of spacecraft. The Nodes satellites accomplished all of their planned mission objectives including three technology ‘firsts’ for small spacecraft: commanding a spacecraft not in direct contact with the ground by crosslinking commands through a space network; crosslinking science data from one Nodes satellite to the second Nodes spacecraft deployed into low Earth orbit satellite before sending it to the ground; and communication, one ultra high frequency autonomous reconfiguration of the space (UHF) radio for crosslink communication, and communications network using the capability an additional UHF beacon radio to transmit of Nodes to automatically select which state-of-health information. satellite is best suited to serve as the ground relay each day. The Nodes science instruments, identical to those on the EDSN satellites, collected The Nodes mission consists of two 1.5- data on the charged particle environment unit (1.5U) CubeSats, each weighing at an altitude of about 250 miles (400 approximately 4.5 pounds (2 kilograms) and kilometers) above Earth. These Energetic measuring about 4 inches x 4 inches x 6.5 Particle Integrating Space Environment inches (10 centimeters x 10 centimeters x Monitor (EPISEM) radiation sensors were 16 centimeters). This mission followed last provided by Montana State University in year’s attempted launch of the eight small Bozeman, Montana, under contract to satellites of the Edison Demonstration of NASA. -

Build a Spacecraft Activity
UAMN Virtual Family Day: Amazing Earth Build a Spacecraft SMAP satellite. Image: NASA. Discover how scientists study Earth from above! Scientists use satellites and spacecraft to study the Earth from outer space. They take pictures of Earth's surface and measure cloud cover, sea levels, glacier movements, and more. Materials Needed: Paper, pencil, craft materials (small recycled boxes, cardboard pieces, paperclips, toothpicks, popsicle sticks, straws, cotton balls, yarn, etc. You can use whatever supplies you have!), fastening materials (glue, tape, rubber bands, string, etc.) Instructions: Step 1: Decide what you want your spacecraft to study. Will it take pictures of clouds? Track forest fires? Measure rainfall? Be creative! Step 2: Design your spacecraft. Draw a picture of what your spacecraft will look like. It will need these parts: Container: To hold everything together. Power Source: To create electricity; solar panels, batteries, etc. Scientific Instruments: This is the why you launched your satellite in the first place! Instruments could include cameras, particle collectors, or magnometers. Communication Device: To relay information back to Earth. Image: NASA SpacePlace. Orientation Finder: A sun or star tracker to show where the spacecraft is pointed. Step 3: Build your spacecraft! Use any craft materials you have available. Let your imagination go wild. Step 4: Your spacecraft will need to survive launching into orbit. Test your spacecraft by gently shaking or spinning it. How well did it hold together? Adjust your design and try again! Model spacecraft examples. Courtesy NASA SpacePlace. Activity adapted from NASA SpacePlace: spaceplace.nasa.gov/build-a-spacecraft/en/ UAMN Virtual Early Explorers: Amazing Earth Studying Earth From Above NASA is best known for exploring outer space, but it also conducts many missions to investigate Earth from above. -

Space Sector Brochure
SPACE SPACE REVOLUTIONIZING THE WAY TO SPACE SPACECRAFT TECHNOLOGIES PROPULSION Moog provides components and subsystems for cold gas, chemical, and electric Moog is a proven leader in components, subsystems, and systems propulsion and designs, develops, and manufactures complete chemical propulsion for spacecraft of all sizes, from smallsats to GEO spacecraft. systems, including tanks, to accelerate the spacecraft for orbit-insertion, station Moog has been successfully providing spacecraft controls, in- keeping, or attitude control. Moog makes thrusters from <1N to 500N to support the space propulsion, and major subsystems for science, military, propulsion requirements for small to large spacecraft. and commercial operations for more than 60 years. AVIONICS Moog is a proven provider of high performance and reliable space-rated avionics hardware and software for command and data handling, power distribution, payload processing, memory, GPS receivers, motor controllers, and onboard computing. POWER SYSTEMS Moog leverages its proven spacecraft avionics and high-power control systems to supply hardware for telemetry, as well as solar array and battery power management and switching. Applications include bus line power to valves, motors, torque rods, and other end effectors. Moog has developed products for Power Management and Distribution (PMAD) Systems, such as high power DC converters, switching, and power stabilization. MECHANISMS Moog has produced spacecraft motion control products for more than 50 years, dating back to the historic Apollo and Pioneer programs. Today, we offer rotary, linear, and specialized mechanisms for spacecraft motion control needs. Moog is a world-class manufacturer of solar array drives, propulsion positioning gimbals, electric propulsion gimbals, antenna positioner mechanisms, docking and release mechanisms, and specialty payload positioners. -

Satellite Orbits
Course Notes for Ocean Colour Remote Sensing Course Erdemli, Turkey September 11 - 22, 2000 Module 1: Satellite Orbits prepared by Assoc Professor Mervyn J Lynch Remote Sensing and Satellite Research Group School of Applied Science Curtin University of Technology PO Box U1987 Perth Western Australia 6845 AUSTRALIA tel +618-9266-7540 fax +618-9266-2377 email <[email protected]> Module 1: Satellite Orbits 1.0 Artificial Earth Orbiting Satellites The early research on orbital mechanics arose through the efforts of people such as Tyco Brahe, Copernicus, Kepler and Galileo who were clearly concerned with some of the fundamental questions about the motions of celestial objects. Their efforts led to the establishment by Keppler of the three laws of planetary motion and these, in turn, prepared the foundation for the work of Isaac Newton who formulated the Universal Law of Gravitation in 1666: namely, that F = GmM/r2 , (1) Where F = attractive force (N), r = distance separating the two masses (m), M = a mass (kg), m = a second mass (kg), G = gravitational constant. It was in the very next year, namely 1667, that Newton raised the possibility of artificial Earth orbiting satellites. A further 300 years lapsed until 1957 when the USSR achieved the first launch into earth orbit of an artificial satellite - Sputnik - occurred. Returning to Newton's equation (1), it would predict correctly (relativity aside) the motion of an artificial Earth satellite if the Earth was a perfect sphere of uniform density, there was no atmosphere or ocean or other external perturbing forces. However, in practice the situation is more complicated and prediction is a less precise science because not all the effects of relevance are accurately known or predictable. -

Microrna-206 Promotes Skeletal Muscle Regeneration and Delays Progression of Duchenne Muscular Dystrophy in Mice
microRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice Ning Liu, … , Rhonda Bassel-Duby, Eric N. Olson J Clin Invest. 2012;122(6):2054-2065. https://doi.org/10.1172/JCI62656. Research Article Muscle biology Skeletal muscle injury activates adult myogenic stem cells, known as satellite cells, to initiate proliferation and differentiation to regenerate new muscle fibers. The skeletal muscle–specific microRNA miR-206 is upregulated in satellite cells following muscle injury, but its role in muscle regeneration has not been defined. Here, we show that miR- 206 promotes skeletal muscle regeneration in response to injury. Genetic deletion of miR-206 in mice substantially delayed regeneration induced by cardiotoxin injury. Furthermore, loss of miR-206 accelerated and exacerbated the dystrophic phenotype in a mouse model of Duchenne muscular dystrophy. We found that miR-206 acts to promote satellite cell differentiation and fusion into muscle fibers through suppressing a collection of negative regulators of myogenesis. Our findings reveal an essential role for miR-206 in satellite cell differentiation during skeletal muscle regeneration and indicate that miR-206 slows progression of Duchenne muscular dystrophy. Find the latest version: https://jci.me/62656/pdf Research article microRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice Ning Liu,1 Andrew H. Williams,1 Johanna M. Maxeiner,1 Svetlana Bezprozvannaya,1 John M. Shelton,2 James A. Richardson,1,3 Rhonda Bassel-Duby,1 and Eric N. Olson1 1Department of Molecular Biology, 2Department of Internal Medicine, and 3Department of Pathology, University of Texas Southwestern Medical Center, Dallas, Texas, USA. -
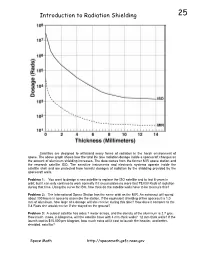
Problem 25, Introduction to Radiation Shielding
Introduction to Radiation Shielding 25 Satellites are designed to withstand many forms of radiation in the harsh environment of space. The above graph shows how the total life time radiation dosage inside a spacecraft changes as the amount of aluminum shielding increases. The data comes from the former MIR space station and the research satellite ISO. The sensitive instruments and electronic systems operate inside the satellite shell and are protected from harmful dosages of radiation by the shielding provided by the spacecraft walls. Problem 1: You want to design a new satellite to replace the ISO satellite and to last 8 years in orbit, but it can only continue to work normally if it accumulates no more that 75,000 Rads of radiation during that time. Using the curve for ISO, how thick do the satellite walls have to be to insure this? Problem 2: The International Space Station has the same orbit as the MIR. An astronaut will spend about 100 hours in space to assemble the station. If the equivalent shielding of her spacesuit is 1.0 mm of aluminum, how large of a dosage will she receive during this time? How does it compare to the 0.4 Rads she would receive if she stayed on the ground? Problem 3: A cubical satellite has sides 1 meter across, and the density of the aluminum is 2.7 g/cc. How much mass, in kilograms, will the satellite have with 4 mm-thick walls? 12 mm-thick walls? If the launch cost is $15,000 per kilogram, how much extra will it cost to launch the heavier, and better- shielded, satellite? Space Math http://spacemath.gsfc.nasa.gov 25 Answer Key: Problem 1: You want to design a new satellite to replace the ISO satellite and to last 8 years in orbit, but it can only continue to work normally if it accumulates no more that 75,000 Rads of radiation during that time. -

SPACE VEHICLE OPERATORS CONCEPT of OPERATIONS a Vision to Transform Ground and Launch Operations
SPACE VEHICLE OPERATORS CONCEPT OF OPERATIONS A Vision to Transform Ground and Launch Operations Future Interagency Range and Spaceport Technologies October 2004 Future Interagency Range and Spaceport Technologies (FIRST) FOREWORD The Future Interagency Range and Spaceport Technologies (FIRST) initiative is a partnership and interagency working group of NASA, the Department of Defense (Air Force Space Command and Office of the Secretary of Defense), and the Federal Aviation Administration. The partnership was established to guide transformation of U.S. ground and space launch operations toward a single, integrated national “system” of space transportation systems that enables low-cost, routine, safe access to space for a variety of applications and markets through technology infusion. This multi-agency consortium is formulating plans to create a national program office that will coordinate individual agency plans to produce an integrated national space transportation system infrastructure comprised of spaceports, ranges, and space and air traffic management systems. A set of concepts of operations, or CONOPS, has been produced to articulate a cohesive interagency vision for this future space transportation system in support of FIRST program formulation efforts. These concepts are intended to guide and support the coordinated development of technologies that allow multiple launch vehicle architectures and missions to be supported by the same ground and launch systems without significant modification. These documents reflect the interests of the partners in the working group, and are not intended to imply final approval or policy of any of the participating agencies. These visionary CONOPS documents have been built on the foundation that was established over the past two years by the Advanced Range Technology Working Group (ARTWG) and Advanced Spaceport Technology Working Group (ASTWG). -

The World's Spaceport
Strict security THE WORLD’S The French government, the CSG, and Arianespace follow strict security measures that meet the most rigorous international SPACEPORT and national agreements and requirements. Arianespace activities are characterized as highly security sensitive ones by the French governement and consequently very strict and rigorous measures are implemented with the Ideal location support of national authorities to satisfy both national and international requirements. They apply to the three launch The Guiana Space Center (CSG) offers ideal conditions for systems: Ariane 5, Soyuz, and Vega, and strictly limit access launching any payload to any orbit at any time. Located to spacecraft. at 5 degrees North latitude, its proximity to the equator provides an extra boost of energy due to the Earth’s Specifically, the security regime is compliant with requirements rotation – a slingshot effect that is greater here than at most governing the export of U.S. manufactured satellites or parts other launch sites. under the ITAR regulation. State-of-the-art facilities Safety mission TECHNICAL OVERVIEW The CSG provides modern Payload Preparation Facilities The CSG entities apply rigorous Safety Rules during each that are recognized for their high quality in the space launch campaign: this includes authorization of equipment industry. The facilities are capable of processing several use, operator certification, and permanent operation spacecrafts from different customers simultaneously, thanks monitoring. Any potentially dangerous activity is to be to vast clean-rooms and commodious infrastructure. reported to the CSG responsible, which in turn, makes certain Designed to support the rockets’ multiple launch capability that safety equipment and emergency response teams are SOYUZ and high launch tempo, the preparation facilities meet the poised to deal with any hazard. -

Satellite Accumulation Areas
Satellite Accumulation Areas Guidance on Hazardous Waste Requirements Introduction Wisconsin businesses and institutions are required by federal and state laws Hazardous waste to manage hazardous waste in accordance with the Resource Conservation regulations are found and Recovery Act requirements. These regulations allow small and large in chs. NR 660-679 quantity generators to manage routinely accumulated hazardous wastes from activities such as process lines or laboratories under satellite of the Wisconsin accumulation area requirements. Administrative Code. Hazardous waste containers in SAA areas must be kept at or near the point of generation of the waste and kept under the control of the operator in charge of the waste generation process. Additionally, although the SAA may have more than one container or waste stream, the area is restricted to accumulating a total of no more than 55 gallons of non-acute hazardous waste, or 1 quart of liquid acute hazardous waste or 1 kilogram of solid acute hazardous waste. [s. NR 662.015 (1), Wis. Adm. Code] Generators must count the hazardous waste accumulated at their SAAs when evaluating their generation totals each calendar month. Accurate generation totals are required to establish the correct generator status of the facility. This Wisconsin Department of Natural Resources provides definitions, general requirements, and common scenarios pertaining to hazardous waste SAAs regulated by the DNR's hazardous waste program. It applies to large and small quantity hazardous waste generators, along with treatment, storage and disposal facilities that are also hazardous waste generators. For further guidance on waste generation, counting monthly totals, and other applicable regulatory requirements, see the Quick Reference Guide (WA-1821). -

10. Spacecraft Configurations MAE 342 2016
2/12/20 Spacecraft Configurations Space System Design, MAE 342, Princeton University Robert Stengel • Angular control approaches • Low-Earth-orbit configurations – Satellite buses – Nanosats/cubesats – Earth resources satellites – Atmospheric science and meteorology satellites – Navigation satellites – Communications satellites – Astronomy satellites – Military satellites – Tethered satellites • Lunar configurations • Deep-space configurations Copyright 2016 by Robert Stengel. All rights reserved. For educational use only. 1 http://www.princeton.edu/~stengel/MAE342.html 1 Angular Attitude of Satellite Configurations • Spinning satellites – Angular attitude maintained by gyroscopic moment • Randomly oriented satellites and magnetic coil – Angular attitude is free to vary – Axisymmetric distribution of mass, solar cells, and instruments Television Infrared Observation (TIROS-7) Orbital Satellite Carrying Amateur Radio (OSCAR-1) ESSA-2 TIROS “Cartwheel” 2 2 1 2/12/20 Attitude-Controlled Satellite Configurations • Dual-spin satellites • Attitude-controlled satellites – Angular attitude maintained by gyroscopic moment and thrusters – Angular attitude maintained by 3-axis control system – Axisymmetric distribution of mass and solar cells – Non-symmetric distribution of mass, solar cells – Instruments and antennas do not spin and instruments INTELSAT-IVA NOAA-17 3 3 LADEE Bus Modules Satellite Buses Standardization of common components for a variety of missions Modular Common Spacecraft Bus Lander Congiguration 4 4 2 2/12/20 Hine et al 5 5 Evolution