Ideal Gas Equation of State.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

Entropy: Ideal Gas Processes
Chapter 19: The Kinec Theory of Gases Thermodynamics = macroscopic picture Gases micro -> macro picture One mole is the number of atoms in 12 g sample Avogadro’s Number of carbon-12 23 -1 C(12)—6 protrons, 6 neutrons and 6 electrons NA=6.02 x 10 mol 12 atomic units of mass assuming mP=mn Another way to do this is to know the mass of one molecule: then So the number of moles n is given by M n=N/N sample A N = N A mmole−mass € Ideal Gas Law Ideal Gases, Ideal Gas Law It was found experimentally that if 1 mole of any gas is placed in containers that have the same volume V and are kept at the same temperature T, approximately all have the same pressure p. The small differences in pressure disappear if lower gas densities are used. Further experiments showed that all low-density gases obey the equation pV = nRT. Here R = 8.31 K/mol ⋅ K and is known as the "gas constant." The equation itself is known as the "ideal gas law." The constant R can be expressed -23 as R = kNA . Here k is called the Boltzmann constant and is equal to 1.38 × 10 J/K. N If we substitute R as well as n = in the ideal gas law we get the equivalent form: NA pV = NkT. Here N is the number of molecules in the gas. The behavior of all real gases approaches that of an ideal gas at low enough densities. Low densitiens m= enumberans tha oft t hemoles gas molecul es are fa Nr e=nough number apa ofr tparticles that the y do not interact with one another, but only with the walls of the gas container. -
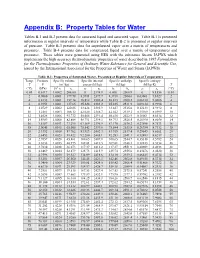
Appendix B: Property Tables for Water
Appendix B: Property Tables for Water Tables B-1 and B-2 present data for saturated liquid and saturated vapor. Table B-1 is presented information at regular intervals of temperature while Table B-2 is presented at regular intervals of pressure. Table B-3 presents data for superheated vapor over a matrix of temperatures and pressures. Table B-4 presents data for compressed liquid over a matrix of temperatures and pressures. These tables were generated using EES with the substance Steam_IAPWS which implements the high accuracy thermodynamic properties of water described in 1995 Formulation for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use, issued by the International Associated for the Properties of Water and Steam (IAPWS). Table B-1: Properties of Saturated Water, Presented at Regular Intervals of Temperature Temp. Pressure Specific volume Specific internal Specific enthalpy Specific entropy T P (m3/kg) energy (kJ/kg) (kJ/kg) (kJ/kg-K) T 3 (°C) (kPa) 10 vf vg uf ug hf hg sf sg (°C) 0.01 0.6117 1.0002 206.00 0 2374.9 0.000 2500.9 0 9.1556 0.01 2 0.7060 1.0001 179.78 8.3911 2377.7 8.3918 2504.6 0.03061 9.1027 2 4 0.8135 1.0001 157.14 16.812 2380.4 16.813 2508.2 0.06110 9.0506 4 6 0.9353 1.0001 137.65 25.224 2383.2 25.225 2511.9 0.09134 8.9994 6 8 1.0729 1.0002 120.85 33.626 2385.9 33.627 2515.6 0.12133 8.9492 8 10 1.2281 1.0003 106.32 42.020 2388.7 42.022 2519.2 0.15109 8.8999 10 12 1.4028 1.0006 93.732 50.408 2391.4 50.410 2522.9 0.18061 8.8514 12 14 1.5989 1.0008 82.804 58.791 2394.1 58.793 2526.5 -

Equation of State of an Ideal Gas
ASME231 Atmospheric Thermodynamics NC A&T State U Department of Physics Dr. Yuh-Lang Lin http://mesolab.org [email protected] Lecture 3: Equation of State of an Ideal Gas As mentioned earlier, the state of a system is the condition of the system (or part of the system) at an instant of time measured by its properties. Those properties are called state variables, such as T, p, and V. The equation which relates T, p, and V is called equation of state. Since they are related by the equation of state, only two of them are independent. Thus, all the other thermodynamic properties will depend on the state defined by two independent variables by state functions. PVT system: The simplest thermodynamic system consists of a fixed mass of a fluid uninfluenced by chemical reactions or external fields. Such a system can be described by the pressure, volume and temperature, which are related by an equation of state f (p, V, T) = 0, (2.1) 1 where only two of them are independent. From physical experiments, the equation of state for an ideal gas, which is defined as a hypothetical gas whose molecules occupy negligible space and have no interactions, may be written as p = RT, (2.2) or p = RT, (2.3) where -3 : density (=m/V) in kg m , 3 -1 : specific volume (=V/m=1/) in m kg . R: specific gas constant (R=R*/M), -1 -1 -1 -1 [R=287 J kg K for dry air (Rd), 461 J kg K for water vapor for water vapor (Rv)]. -

Chapter 3 Equations of State
Chapter 3 Equations of State The simplest way to derive the Helmholtz function of a fluid is to directly integrate the equation of state with respect to volume (Sadus, 1992a, 1994). An equation of state can be applied to either vapour-liquid or supercritical phenomena without any conceptual difficulties. Therefore, in addition to liquid-liquid and vapour -liquid properties, it is also possible to determine transitions between these phenomena from the same inputs. All of the physical properties of the fluid except ideal gas are also simultaneously calculated. Many equations of state have been proposed in the literature with either an empirical, semi- empirical or theoretical basis. Comprehensive reviews can be found in the works of Martin (1979), Gubbins (1983), Anderko (1990), Sandler (1994), Economou and Donohue (1996), Wei and Sadus (2000) and Sengers et al. (2000). The van der Waals equation of state (1873) was the first equation to predict vapour-liquid coexistence. Later, the Redlich-Kwong equation of state (Redlich and Kwong, 1949) improved the accuracy of the van der Waals equation by proposing a temperature dependence for the attractive term. Soave (1972) and Peng and Robinson (1976) proposed additional modifications of the Redlich-Kwong equation to more accurately predict the vapour pressure, liquid density, and equilibria ratios. Guggenheim (1965) and Carnahan and Starling (1969) modified the repulsive term of van der Waals equation of state and obtained more accurate expressions for hard sphere systems. Christoforakos and Franck (1986) modified both the attractive and repulsive terms of van der Waals equation of state. Boublik (1981) extended the Carnahan-Starling hard sphere term to obtain an accurate equation for hard convex geometries. -

IB Questionbank
Topic 3 Past Paper [94 marks] This question is about thermal energy transfer. A hot piece of iron is placed into a container of cold water. After a time the iron and water reach thermal equilibrium. The heat capacity of the container is negligible. specific heat capacity. [2 marks] 1a. Define Markscheme the energy required to change the temperature (of a substance) by 1K/°C/unit degree; of mass 1 kg / per unit mass; [5 marks] 1b. The following data are available. Mass of water = 0.35 kg Mass of iron = 0.58 kg Specific heat capacity of water = 4200 J kg–1K–1 Initial temperature of water = 20°C Final temperature of water = 44°C Initial temperature of iron = 180°C (i) Determine the specific heat capacity of iron. (ii) Explain why the value calculated in (b)(i) is likely to be different from the accepted value. Markscheme (i) use of mcΔT; 0.58×c×[180-44]=0.35×4200×[44-20]; c=447Jkg-1K-1≈450Jkg-1K-1; (ii) energy would be given off to surroundings/environment / energy would be absorbed by container / energy would be given off through vaporization of water; hence final temperature would be less; hence measured value of (specific) heat capacity (of iron) would be higher; This question is in two parts. Part 1 is about ideal gases and specific heat capacity. Part 2 is about simple harmonic motion and waves. Part 1 Ideal gases and specific heat capacity State assumptions of the kinetic model of an ideal gas. [2 marks] 2a. two Markscheme point molecules / negligible volume; no forces between molecules except during contact; motion/distribution is random; elastic collisions / no energy lost; obey Newton’s laws of motion; collision in zero time; gravity is ignored; [4 marks] 2b. -

How Compressible Are Innovation Processes?
1 How Compressible are Innovation Processes? Hamid Ghourchian, Arash Amini, Senior Member, IEEE, and Amin Gohari, Senior Member, IEEE Abstract The sparsity and compressibility of finite-dimensional signals are of great interest in fields such as compressed sensing. The notion of compressibility is also extended to infinite sequences of i.i.d. or ergodic random variables based on the observed error in their nonlinear k-term approximation. In this work, we use the entropy measure to study the compressibility of continuous-domain innovation processes (alternatively known as white noise). Specifically, we define such a measure as the entropy limit of the doubly quantized (time and amplitude) process. This provides a tool to compare the compressibility of various innovation processes. It also allows us to identify an analogue of the concept of “entropy dimension” which was originally defined by R´enyi for random variables. Particular attention is given to stable and impulsive Poisson innovation processes. Here, our results recognize Poisson innovations as the more compressible ones with an entropy measure far below that of stable innovations. While this result departs from the previous knowledge regarding the compressibility of fat-tailed distributions, our entropy measure ranks stable innovations according to their tail decay. Index Terms Compressibility, entropy, impulsive Poisson process, stable innovation, white L´evy noise. I. INTRODUCTION The compressible signal models have been extensively used to represent or approximate various types of data such as audio, image and video signals. The concept of compressibility has been separately studied in information theory and signal processing societies. In information theory, this concept is usually studied via the well-known entropy measure and its variants. -

Chapter 6 - Engineering Equations of State II
Introductory Chemical Engineering Thermodynamics By J.R. Elliott and C.T. Lira Chapter 6 - Engineering Equations of State II. Generalized Fluid Properties The principle of two-parameter corresponding states 50 50 40 40 30 30 T=705K 20 20 ρ T=286K L 10 10 T=470 T=191K T=329K 0 0 0 0.1 0.2 0.3 0.4 0 0.1 0.2 0.3 0.4 0.5 -10 T=133K -10 ρV Density (g/cc) Density (g/cc) VdW Pressure (bars) in Methane VdW Pressure (bars) in Pentane Critical Definitions: Tc - critical temperature - the temperature above which no liquid can exist. Pc - critical pressure - the pressure above which no vapor can exist. ω - acentric factor - a third parameter which helps to specify the vapor pressure curve which, in turn, affects the rest of the thermodynamic variables. ∂ ∂ 2 P = P = 0 and 0 at Tc and Pc Note: at the critical point, ∂ρ ∂ρ 2 T T Chapter 6 - Engineering Equations of State Slide 1 II. Generalized Fluid Properties The van der Waals (1873) Equation Of State (vdW-EOS) Based on some semi-empirical reasoning about the ways that temperature and density affect the pressure, van der Waals (1873) developed the equation below, which he considered to be fairly crude. We will discuss the reasoning at the end of the chapter, but it is useful to see what the equations are and how we use them before deriving the details. The vdW-EOS is: bρ aρ 1 aρ Z =+1 −= − ()1− bρ RT()1− bρ RT van der Waals’ trick for characterizing the difference between subcritical and supercritical fluids was to recognize that, at the critical point, ∂P ∂ 2 P = 0 and = 0 at T , P ∂ρ ∂ρ 2 c c T T Since there are only two “undetermined parameters” in his EOS (a and b), he has reduced the problem to one of two equations and two unknowns. -

Thermodynamics
ME346A Introduction to Statistical Mechanics { Wei Cai { Stanford University { Win 2011 Handout 6. Thermodynamics January 26, 2011 Contents 1 Laws of thermodynamics 2 1.1 The zeroth law . .3 1.2 The first law . .4 1.3 The second law . .5 1.3.1 Efficiency of Carnot engine . .5 1.3.2 Alternative statements of the second law . .7 1.4 The third law . .8 2 Mathematics of thermodynamics 9 2.1 Equation of state . .9 2.2 Gibbs-Duhem relation . 11 2.2.1 Homogeneous function . 11 2.2.2 Virial theorem / Euler theorem . 12 2.3 Maxwell relations . 13 2.4 Legendre transform . 15 2.5 Thermodynamic potentials . 16 3 Worked examples 21 3.1 Thermodynamic potentials and Maxwell's relation . 21 3.2 Properties of ideal gas . 24 3.3 Gas expansion . 28 4 Irreversible processes 32 4.1 Entropy and irreversibility . 32 4.2 Variational statement of second law . 32 1 In the 1st lecture, we will discuss the concepts of thermodynamics, namely its 4 laws. The most important concepts are the second law and the notion of Entropy. (reading assignment: Reif x 3.10, 3.11) In the 2nd lecture, We will discuss the mathematics of thermodynamics, i.e. the machinery to make quantitative predictions. We will deal with partial derivatives and Legendre transforms. (reading assignment: Reif x 4.1-4.7, 5.1-5.12) 1 Laws of thermodynamics Thermodynamics is a branch of science connected with the nature of heat and its conver- sion to mechanical, electrical and chemical energy. (The Webster pocket dictionary defines, Thermodynamics: physics of heat.) Historically, it grew out of efforts to construct more efficient heat engines | devices for ex- tracting useful work from expanding hot gases (http://www.answers.com/thermodynamics). -

On the Van Der Waals Gas, Contact Geometry and the Toda Chain
entropy Article On the van der Waals Gas, Contact Geometry and the Toda Chain Diego Alarcón, P. Fernández de Córdoba ID , J. M. Isidro * ID and Carlos Orea Instituto Universitario de Matemática Pura y Aplicada, Universidad Politécnica de Valencia, 46022 Valencia, Spain; [email protected] (D.A.); [email protected] (P.F.d.C.); [email protected] (C.O.) * Correspondence: [email protected] Received: 7 June 2018; Accepted: 16 July 2018; Published: 26 July 2018 Abstract: A Toda–chain symmetry is shown to underlie the van der Waals gas and its close cousin, the ideal gas. Links to contact geometry are explored. Keywords: van der Waals gas; contact geometry; Toda chain 1. Introduction The contact geometry of the classical van der Waals gas [1] is described geometrically using a five-dimensional contact manifold M [2] that can be endowed with the local coordinates U (internal energy), S (entropy), V (volume), T (temperature) and p (pressure). This description corresponds to a choice of the fundamental equation, in the energy representation, in which U depends on the two extensive variables S and V. One defines the corresponding momenta T = ¶U/¶S and −p = ¶U/¶V. Then, the standard contact form on M reads [3,4] a = dU + TdS − pdV. (1) One can introduce Poisson brackets on the four-dimensional Poisson manifold P (a submanifold of M) spanned by the coordinates S, V and their conjugate variables T, −p, the nonvanishing brackets being fS, Tg = 1, fV, −pg = 1. (2) Given now an equation of state f (p, T,...) = 0, (3) one can make the replacements T = ¶U/¶S, −p = ¶U/¶V in order to obtain ¶U ¶U f − , ,.. -

Thermodynamics of Ideal Gases
D Thermodynamics of ideal gases An ideal gas is a nice “laboratory” for understanding the thermodynamics of a fluid with a non-trivial equation of state. In this section we shall recapitulate the conventional thermodynamics of an ideal gas with constant heat capacity. For more extensive treatments, see for example [67, 66]. D.1 Internal energy In section 4.1 we analyzed Bernoulli’s model of a gas consisting of essentially 1 2 non-interacting point-like molecules, and found the pressure p = 3 ½ v where v is the root-mean-square average molecular speed. Using the ideal gas law (4-26) the total molecular kinetic energy contained in an amount M = ½V of the gas becomes, 1 3 3 Mv2 = pV = nRT ; (D-1) 2 2 2 where n = M=Mmol is the number of moles in the gas. The derivation in section 4.1 shows that the factor 3 stems from the three independent translational degrees of freedom available to point-like molecules. The above formula thus expresses 1 that in a mole of a gas there is an internal kinetic energy 2 RT associated with each translational degree of freedom of the point-like molecules. Whereas monatomic gases like argon have spherical molecules and thus only the three translational degrees of freedom, diatomic gases like nitrogen and oxy- Copyright °c 1998{2004, Benny Lautrup Revision 7.7, January 22, 2004 662 D. THERMODYNAMICS OF IDEAL GASES gen have stick-like molecules with two extra rotational degrees of freedom or- thogonally to the bridge connecting the atoms, and multiatomic gases like carbon dioxide and methane have the three extra rotational degrees of freedom. -

An Entropy-Stable Hybrid Scheme for Simulations of Transcritical Real-Fluid
Journal of Computational Physics 340 (2017) 330–357 Contents lists available at ScienceDirect Journal of Computational Physics www.elsevier.com/locate/jcp An entropy-stable hybrid scheme for simulations of transcritical real-fluid flows Peter C. Ma, Yu Lv ∗, Matthias Ihme Department of Mechanical Engineering, Stanford University, Stanford, CA 94305, USA a r t i c l e i n f o a b s t r a c t Article history: A finite-volume method is developed for simulating the mixing of turbulent flows at Received 30 August 2016 transcritical conditions. Spurious pressure oscillations associated with fully conservative Received in revised form 9 March 2017 formulations are addressed by extending a double-flux model to real-fluid equations of Accepted 12 March 2017 state. An entropy-stable formulation that combines high-order non-dissipative and low- Available online 22 March 2017 order dissipative finite-volume schemes is proposed to preserve the physical realizability Keywords: of numerical solutions across large density gradients. Convexity conditions and constraints Real fluid on the application of the cubic state equation to transcritical flows are investigated, and Spurious pressure oscillations conservation properties relevant to the double-flux model are examined. The resulting Double-flux model method is applied to a series of test cases to demonstrate the capability in simulations Entropy stable of problems that are relevant for multi-species transcritical real-fluid flows. Hybrid scheme © 2017 Elsevier Inc. All rights reserved. 1. Introduction The accurate and robust simulation of transcritical real-fluid effects is crucial for many engineering applications, such as fuel injection in internal-combustion engines, rocket motors and gas turbines.