Toward Evidence-Based Medical Statistics. 2: the Bayes Factor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Arxiv:1803.00360V2 [Stat.CO] 2 Mar 2018 Prone to Misinterpretation [2, 3]](https://docslib.b-cdn.net/cover/7623/arxiv-1803-00360v2-stat-co-2-mar-2018-prone-to-misinterpretation-2-3-297623.webp)
Arxiv:1803.00360V2 [Stat.CO] 2 Mar 2018 Prone to Misinterpretation [2, 3]
Computing Bayes factors to measure evidence from experiments: An extension of the BIC approximation Thomas J. Faulkenberry∗ Tarleton State University Bayesian inference affords scientists with powerful tools for testing hypotheses. One of these tools is the Bayes factor, which indexes the extent to which support for one hypothesis over another is updated after seeing the data. Part of the hesitance to adopt this approach may stem from an unfamiliarity with the computational tools necessary for computing Bayes factors. Previous work has shown that closed form approximations of Bayes factors are relatively easy to obtain for between-groups methods, such as an analysis of variance or t-test. In this paper, I extend this approximation to develop a formula for the Bayes factor that directly uses infor- mation that is typically reported for ANOVAs (e.g., the F ratio and degrees of freedom). After giving two examples of its use, I report the results of simulations which show that even with minimal input, this approximate Bayes factor produces similar results to existing software solutions. Note: to appear in Biometrical Letters. I. INTRODUCTION A. The Bayes factor Bayesian inference is a method of measurement that is Hypothesis testing is the primary tool for statistical based on the computation of P (H j D), which is called inference across much of the biological and behavioral the posterior probability of a hypothesis H, given data D. sciences. As such, most scientists are trained in classical Bayes' theorem casts this probability as null hypothesis significance testing (NHST). The scenario for testing a hypothesis is likely familiar to most readers of this journal. -

1 Estimation and Beyond in the Bayes Universe
ISyE8843A, Brani Vidakovic Handout 7 1 Estimation and Beyond in the Bayes Universe. 1.1 Estimation No Bayes estimate can be unbiased but Bayesians are not upset! No Bayes estimate with respect to the squared error loss can be unbiased, except in a trivial case when its Bayes’ risk is 0. Suppose that for a proper prior ¼ the Bayes estimator ±¼(X) is unbiased, Xjθ (8θ)E ±¼(X) = θ: This implies that the Bayes risk is 0. The Bayes risk of ±¼(X) can be calculated as repeated expectation in two ways, θ Xjθ 2 X θjX 2 r(¼; ±¼) = E E (θ ¡ ±¼(X)) = E E (θ ¡ ±¼(X)) : Thus, conveniently choosing either EθEXjθ or EX EθjX and using the properties of conditional expectation we have, θ Xjθ 2 θ Xjθ X θjX X θjX 2 r(¼; ±¼) = E E θ ¡ E E θ±¼(X) ¡ E E θ±¼(X) + E E ±¼(X) θ Xjθ 2 θ Xjθ X θjX X θjX 2 = E E θ ¡ E θ[E ±¼(X)] ¡ E ±¼(X)E θ + E E ±¼(X) θ Xjθ 2 θ X X θjX 2 = E E θ ¡ E θ ¢ θ ¡ E ±¼(X)±¼(X) + E E ±¼(X) = 0: Bayesians are not upset. To check for its unbiasedness, the Bayes estimator is averaged with respect to the model measure (Xjθ), and one of the Bayesian commandments is: Thou shall not average with respect to sample space, unless you have Bayesian design in mind. Even frequentist agree that insisting on unbiasedness can lead to bad estimators, and that in their quest to minimize the risk by trading off between variance and bias-squared a small dosage of bias can help. -

Hierarchical Models & Bayesian Model Selection
Hierarchical Models & Bayesian Model Selection Geoffrey Roeder Departments of Computer Science and Statistics University of British Columbia Jan. 20, 2016 Geoffrey Roeder Hierarchical Models & Bayesian Model Selection Jan. 20, 2016 Contact information Please report any typos or errors to geoff[email protected] Geoffrey Roeder Hierarchical Models & Bayesian Model Selection Jan. 20, 2016 Outline 1 Hierarchical Bayesian Modelling Coin toss redux: point estimates for θ Hierarchical models Application to clinical study 2 Bayesian Model Selection Introduction Bayes Factors Shortcut for Marginal Likelihood in Conjugate Case Geoffrey Roeder Hierarchical Models & Bayesian Model Selection Jan. 20, 2016 Outline 1 Hierarchical Bayesian Modelling Coin toss redux: point estimates for θ Hierarchical models Application to clinical study 2 Bayesian Model Selection Introduction Bayes Factors Shortcut for Marginal Likelihood in Conjugate Case Geoffrey Roeder Hierarchical Models & Bayesian Model Selection Jan. 20, 2016 Let Y be a random variable denoting number of observed heads in n coin tosses. Then, we can model Y ∼ Bin(n; θ), with probability mass function n p(Y = y j θ) = θy (1 − θ)n−y (1) y We want to estimate the parameter θ Coin toss: point estimates for θ Probability model Consider the experiment of tossing a coin n times. Each toss results in heads with probability θ and tails with probability 1 − θ Geoffrey Roeder Hierarchical Models & Bayesian Model Selection Jan. 20, 2016 We want to estimate the parameter θ Coin toss: point estimates for θ Probability model Consider the experiment of tossing a coin n times. Each toss results in heads with probability θ and tails with probability 1 − θ Let Y be a random variable denoting number of observed heads in n coin tosses. -

Bayes Factors in Practice Author(S): Robert E
Bayes Factors in Practice Author(s): Robert E. Kass Source: Journal of the Royal Statistical Society. Series D (The Statistician), Vol. 42, No. 5, Special Issue: Conference on Practical Bayesian Statistics, 1992 (2) (1993), pp. 551-560 Published by: Blackwell Publishing for the Royal Statistical Society Stable URL: http://www.jstor.org/stable/2348679 Accessed: 10/08/2010 18:09 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=black. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Blackwell Publishing and Royal Statistical Society are collaborating with JSTOR to digitize, preserve and extend access to Journal of the Royal Statistical Society. -

Bayes Factor Consistency
Bayes Factor Consistency Siddhartha Chib John M. Olin School of Business, Washington University in St. Louis and Todd A. Kuffner Department of Mathematics, Washington University in St. Louis July 1, 2016 Abstract Good large sample performance is typically a minimum requirement of any model selection criterion. This article focuses on the consistency property of the Bayes fac- tor, a commonly used model comparison tool, which has experienced a recent surge of attention in the literature. We thoroughly review existing results. As there exists such a wide variety of settings to be considered, e.g. parametric vs. nonparametric, nested vs. non-nested, etc., we adopt the view that a unified framework has didactic value. Using the basic marginal likelihood identity of Chib (1995), we study Bayes factor asymptotics by decomposing the natural logarithm of the ratio of marginal likelihoods into three components. These are, respectively, log ratios of likelihoods, prior densities, and posterior densities. This yields an interpretation of the log ra- tio of posteriors as a penalty term, and emphasizes that to understand Bayes factor consistency, the prior support conditions driving posterior consistency in each respec- tive model under comparison should be contrasted in terms of the rates of posterior contraction they imply. Keywords: Bayes factor; consistency; marginal likelihood; asymptotics; model selection; nonparametric Bayes; semiparametric regression. 1 1 Introduction Bayes factors have long held a special place in the Bayesian inferential paradigm, being the criterion of choice in model comparison problems for such Bayesian stalwarts as Jeffreys, Good, Jaynes, and others. An excellent introduction to Bayes factors is given by Kass & Raftery (1995). -

Calibrated Bayes Factors for Model Selection and Model Averaging
Calibrated Bayes factors for model selection and model averaging Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Pingbo Lu, B.S. Graduate Program in Statistics The Ohio State University 2012 Dissertation Committee: Dr. Steven N. MacEachern, Co-Advisor Dr. Xinyi Xu, Co-Advisor Dr. Christopher M. Hans ⃝c Copyright by Pingbo Lu 2012 Abstract The discussion of statistical model selection and averaging has a long history and is still attractive nowadays. Arguably, the Bayesian ideal of model comparison based on Bayes factors successfully overcomes the difficulties and drawbacks for which frequen- tist hypothesis testing has been criticized. By putting prior distributions on model- specific parameters, Bayesian models will be completed hierarchically and thus many statistical summaries, such as posterior model probabilities, model-specific posteri- or distributions and model-average posterior distributions can be derived naturally. These summaries enable us to compare inference summaries such as the predictive distributions. Importantly, a Bayes factor between two models can be greatly affected by the prior distributions on the model parameters. When prior information is weak, very dispersed proper prior distributions are often used. This is known to create a problem for the Bayes factor when competing models that differ in dimension, and it is of even greater concern when one of the models is of infinite dimension. Therefore, we propose an innovative criterion called the calibrated Bayes factor, which uses training samples to calibrate the prior distributions so that they achieve a reasonable level of "information". The calibrated Bayes factor is then computed as the Bayes factor over the remaining data. -
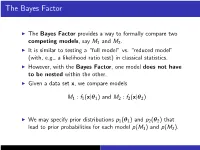
The Bayes Factor
The Bayes Factor I The Bayes Factor provides a way to formally compare two competing models, say M1 and M2. I It is similar to testing a “full model” vs. “reduced model” (with, e.g., a likelihood ratio test) in classical statistics. I However, with the Bayes Factor, one model does not have to be nested within the other. I Given a data set x, we compare models M1 : f1(x|θ1) and M2 : f2(x|θ2) I We may specify prior distributions p1(θ1) and p2(θ2) that lead to prior probabilities for each model p(M1) and p(M2). The Bayes Factor By Bayes’ Law, the posterior odds in favor of Model 1 versus Model 2 is: R p(M1)f1(x|θ1)p1(θ1) dθ1 π(M |x) Θ1 p(x) 1 = π(M2|x) R p(M2)f2(x|θ2)p2(θ2) dθ2 Θ2 p(x) R f (x|θ )p (θ ) dθ p(M1) Θ1 1 1 1 1 1 = · R p(M2) f (x|θ )p (θ ) dθ Θ2 2 2 2 2 2 = [prior odds] × [Bayes Factor B(x)] The Bayes Factor Rearranging, the Bayes Factor is: π(M |x) p(M ) B(x) = 1 × 2 π(M2|x) p(M1) π(M |x)/π(M |x) = 1 2 p(M1)/p(M2) (the ratio of the posterior odds for M1 to the prior odds for M1). The Bayes Factor I Note: If the prior model probabilities are equal, i.e., p(M1) = p(M2), then the Bayes Factor equals the posterior odds for M1. -

On the Derivation of the Bayesian Information Criterion
On the derivation of the Bayesian Information Criterion H. S. Bhat∗† N. Kumar∗ November 8, 2010 Abstract We present a careful derivation of the Bayesian Inference Criterion (BIC) for model selection. The BIC is viewed here as an approximation to the Bayes Factor. One of the main ingredients in the approximation, the use of Laplace’s method for approximating integrals, is explained well in the literature. Our derivation sheds light on this and other steps in the derivation, such as the use of a flat prior and the invocation of the weak law of large numbers, that are not often discussed in detail. 1 Notation Let us define the notation that we will use: y : observed data y1,...,yn Mi : candidate model P (y|Mi) : marginal likelihood of the model Mi given the data θi : vector of parameters in the model Mi gi(θi) : the prior density of the parameters θi f(y|θi) : the density of the data given the parameters θi L(θi|y) : the likelihood of y given the model Mi θˆi : the MLE of θi that maximizes L(θi|y) 2 Bayes Factor The Bayesian approach to model selection [1] is to maximize the posterior probability of n a model (Mi) given the data {yj}j=1. Applying Bayes theorem to calculate the posterior ∗School of Natural Sciences, University of California, Merced, 5200 N. Lake Rd., Merced, CA 95343 †Corresponding author, email: [email protected] 1 probability of a model given the data, we get P (y1,...,yn|Mi)P (Mi) P (Mi|y1,...,yn)= , (1) P (y1,...,yn) where P (y1,...,yn|Mi) is called the marginal likelihood of the model Mi. -

Bayesian Inference Chapter 5: Model Selection
Bayesian Inference Chapter 5: Model selection Conchi Aus´ınand Mike Wiper Department of Statistics Universidad Carlos III de Madrid Master in Business Administration and Quantitative Methodsl Master in Mathematical Engineering Conchi Aus´ınand Mike Wiper Model selection Masters Programmes 1 / 33 Objective In this class we consider problems where we have various contending models and show how these can be compared and also look at the possibilities of model averaging. Conchi Aus´ınand Mike Wiper Model selection Masters Programmes 2 / 33 Basics In principle, model selection is easy. Suppose that we wish to compare two models, M1, M2. Then, define prior probabilities, P(M1) and P(M2) = 1 − P(M1). Given data, we can calculate the posterior probabilities via Bayes theorem: f (xjM )P(M ) P(M jx) = 1 1 1 f (x) where f (x) = f (xjM1)P(M1) + f (xjM2)P(M2). Note also that if model Mi is parametric with parameters θi , then Z f (xjMi ) = f (xjMi ; θi )f (θi jMi ) dθi Conchi Aus´ınand Mike Wiper Model selection Masters Programmes 3 / 33 Now consider the possible losses (negative utilities) associated with taking a wrong decision. L(select M2jM1 true) and L(select M1jM2 true): (something like type I and type II errors) Take the decision which minimizes the expected loss (Bayes decision). Expected loss for choosing M1 is P(M2jx)L(M1jM2) and similarly for M2. If the loss functions are equal, then we just select the model with higher probability. Setting L(M1jM2) = 0:05 and L(M2jM1) = 0:95 means we select M1 if P(M1jx) > 0:05. -

Bayesian Alternatives for Common Null-Hypothesis Significance Tests
Quintana and Eriksen Bayesian alternatives for common null-hypothesis significance tests in psychiatry: A non-technical guide using JASP Daniel S Quintana* and Jon Alm Eriksen *Correspondence: [email protected] Abstract NORMENT, KG Jebsen Centre for Psychosis Research, Division of Despite its popularity, null hypothesis significance testing (NHST) has several Mental Health and Addiction, limitations that restrict statistical inference. Bayesian analysis can address many University of Oslo and Oslo of these limitations, however, this approach has been underutilized largely due to University Hospital, Oslo, Norway Full list of author information is a dearth of accessible software options. JASP is a new open-source statistical available at the end of the article package that facilitates both Bayesian and NHST analysis using a graphical interface. The aim of this article is to provide an applied introduction to Bayesian inference using JASP. First, we will provide a brief background outlining the benefits of Bayesian inference. We will then use JASP to demonstrate Bayesian alternatives for several common null hypothesis significance tests. While Bayesian analysis is by no means a new method, this type of statistical inference has been largely inaccessible for most psychiatry researchers. JASP provides a straightforward means of performing Bayesian analysis using a graphical “point and click” environment that will be familiar to researchers conversant with other graphical statistical packages, such as SPSS. Keywords: Bayesian statistics; null hypothesis significance tests; p-values; statistics; software Consider the development of a new drug to treat social dysfunction. Before reaching the public, scientists need to demonstrate drug efficacy by performing a randomized- controlled trial (RCT) whereby participants are randomized to receive the drug or placebo. -

Bayesian Model Selection
Bayesian model selection Consider the regression problem, where we want to predict the values of an unknown function d N y : R ! R given examples D = (xi; yi) i=1 to serve as training data. In Bayesian linear regression, we made the following assumption about y(x): y(x) = φ(x)>w + "(x); (1) where φ(x) is a now explicitly-written feature expansion of x. We proceed in the normal Bayesian way: we place Gaussian priors on our unknowns, the parameters w and the residuals ", then derive the posterior distribution over w given D, which we use to make predictions. One question left unanswered is how to choose a good feature expansion function φ(x). For example, a purely linear model could use φ(x) = [1; x]>, whereas a quadratic model could use φ(x) = [1; x; x2]>, etc. In general, arbitrary feature expansions φ are allowed. How can I select between them? Even more generally, how do I select whether I should use linear regression or a completely dierent probabilistic model to explain my data? These are questions of model selection, and naturally there is a Bayesian approach to it. Before we continue our discussion of model selection, we will rst dene the word model, which is often used loosely without explicit denition. A model is a parametric family of probability distributions, each of which can explain the observed data. Another way to explain the concept of a model is that if we have chosen a likelihood p(D j θ) for our data, which depends on a parameter θ, then the model is the set of all likelihoods (each one of which is a distribution over D) for every possible value of the parameter θ. -

A Tutorial on Conducting and Interpreting a Bayesian ANOVA in JASP
A Tutorial on Conducting and Interpreting a Bayesian ANOVA in JASP Don van den Bergh∗1, Johnny van Doorn1, Maarten Marsman1, Tim Draws1, Erik-Jan van Kesteren4, Koen Derks1,2, Fabian Dablander1, Quentin F. Gronau1, Simonˇ Kucharsk´y1, Akash R. Komarlu Narendra Gupta1, Alexandra Sarafoglou1, Jan G. Voelkel5, Angelika Stefan1, Alexander Ly1,3, Max Hinne1, Dora Matzke1, and Eric-Jan Wagenmakers1 1University of Amsterdam 2Nyenrode Business University 3Centrum Wiskunde & Informatica 4Utrecht University 5Stanford University Abstract Analysis of variance (ANOVA) is the standard procedure for statistical inference in factorial designs. Typically, ANOVAs are executed using fre- quentist statistics, where p-values determine statistical significance in an all-or-none fashion. In recent years, the Bayesian approach to statistics is increasingly viewed as a legitimate alternative to the p-value. How- ever, the broad adoption of Bayesian statistics {and Bayesian ANOVA in particular{ is frustrated by the fact that Bayesian concepts are rarely taught in applied statistics courses. Consequently, practitioners may be unsure how to conduct a Bayesian ANOVA and interpret the results. Here we provide a guide for executing and interpreting a Bayesian ANOVA with JASP, an open-source statistical software program with a graphical user interface. We explain the key concepts of the Bayesian ANOVA using two empirical examples. ∗Correspondence concerning this article should be addressed to: Don van den Bergh University of Amsterdam, Department of Psychological Methods Postbus 15906, 1001 NK Amsterdam, The Netherlands E-Mail should be sent to: [email protected]. 1 Ubiquitous across the empirical sciences, analysis of variance (ANOVA) al- lows researchers to assess the effects of categorical predictors on a continuous outcome variable.