Bumblebees Can Detect Floral Humidity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SRGC BULB LOG DIARY---Pictures and Text © Ian Young
SRGC ----- Bulb Log Diary ----- ISSN 2514-6114 Pictures and text © Ian Young BULB LOG 24.....................13th June 2018 Meconopsis baileyi Gardeners are always talking about the plants we “grow” however the plants grow themselves, we have to either choose those that will grow in our conditions or make such adaptions to the habitat to allow them to grow. All the Meconopsis baileyi in these pictures seeded themselves and so choose where they would grow just as they would in nature. Most plants produce large numbers of seeds that when ripe are scattered randomly; many will fall on ‘stony ground’ but some will drop onto favourable growing conditions where they will thrive. A valuable lesson I learned early on was that a good gardener is a gardener who discovers which plants grow well for them. Of course we all want to extend the number of plants we grow and take on some of the challenging ones but you will make life easier and your garden better if the core of your planting has plants that are easy in your conditions. Now many of you in hotter drier areas will be looking with envy at the way these Meconopsis seed around and grow so well here but I can assure you that I look back with equal envy at some of the plants that prefer your hotter drier conditions and that we cannot grow. Papaver cambricum (syn. Meconopsis cambrica) Some plants seem able to adapt to most growing conditions and Papaver cambricum is among those in fact it grows so well that many a gardener designates it a weed and tries to eradicate it. -

Diversity and Distribution of the Genus Meconopsis Vig . (Papaveraceae) in the Indian Himalayan Region
Pleione 14(2): 323 - 329. 2020. ISSN: 0973-9467 © East Himalayan Society for Spermatophyte Taxonomy doi:10.26679/Pleione.14.2.2020.323-329 Diversity and distribution of the genus Meconopsis Vig . (Papaveraceae) in the Indian Himalayan region K.S. Kanwal G.B. Pant National Institute of Himalayan Environment (NIHE), Himachal Regional Centre, Mohal Kullu, Himachal Pradesh – 175 126, India E-mail: [email protected] Abstract The present work provides a comprehensive overview of status and distribution of genus Meconopsis Vig .( Papaveraceae) in the Indian Himalayan Region (IHR). Critical assessment of published literature and herbarium records on the diversity, distribution and endemism of the genus Meconopsis Vig. have been carried for the review. A total 22 taxa of Meconopsis is recorded from the IHR, out of which only 7 are reported to the Western Himalayan region (Uttarakhand, Himachal Pradesh, Jammu & Kashmir & Ladakh UT), whereas Eastern Himalaya (Arunachal Pradesh, Sikkim, West Bengal (Darjeeling Hills) is enriched with 19 taxa and 5 taxa are observed common for Western and Eastern Himalayan region. Highest Meconopsis diversity was observed in Arucnhal Pradesh (13 taxa) followed by Sikkim (11 taxa). Several new taxa viz. Meconopsis gakyidiana, M. prainiana var. prainiana, M. prainiana var. lutea, M. merakensis var. merakensis, M. merakensis var. albolutea and M. baileyi subsp. Pratensis are recently recorded from Arunchal Pradesh. The Meconopsis taxa are mainly distributed in the alpine ecosystem ranging from 3000-4200 masl. Conservation strategy is also proposed for protection of Meconopsis taxa in the IHR. Key words Meconopsis, Papaveraceae, Distribution, Indian Himalayan Region, Conservation INTRODUCTION European species, Papaver cambricum L. -

Lectotypification of Papaver Cambricum L. (Papaveraceae)
Lectotypification of Papaver cambricum L. (Papaveraceae) P. Pablo Ferrer-Gallego Abstract FERRER-GALLEGO, P. P. (2015). Lectotypification of Papaver cambricum L. (Papaveraceae). Candollea 70: 207-210. In English, English abstract. DOI: http://dx.doi.org/10.15553/c2015v702a5 The typification of the name Papaver cambricum L. (Papaveraceae) is discussed. This species was previously accepted in the genus Meconopsis Vig. as Meconopsis cambrica (L.) Vig. The protologue of the name and the original material are evaluated. A specimen from the Burser Herbarium (UPS-BURSER) is designated as the lectotype. Keywords PAPAVERACEAE − Meconopsis − Papaver − Lectotypification Address of the author : CIEF, Centro para la Investigación y la Experimentación Forestal, Servicio de Vida Silvestre, Generalitat Valenciana, Avda. Comarques del País Valencià, 114, 46930, Quart de Poblet, Valencia, Spain. E-mail: [email protected] Submitted on October 6, 2014. Accepted on June 9, 2015 Edited by P. Bungener ISSN: 0373-2967 – Online ISSN: 2235-3658 – Candollea 70(2) : 207-210 (2015) © CONSERVATOIRE ET JARDIN BOTANIQUES DE GENÈVE 2015 208 – Lectotypication in Papaver (Papaveraceae) Candollea 70, 2015 Introduction fragments, one with a flower, the other with a flower bud, The genus Meconopsis Vig. (Papaveraceae) comprises approxi- and a label with the annotation “Papaver erraticum pyrenai- mately 70 perennial monocarpic or polycarpic herbs, dis- cum flore flavo Bauh. / In Pyrenaeis” (see Savage, 1937: 27). tributed primarily in southern central Asia (Grey-Wilson, No further original material have been found in any of the 2014). The western European M. cambrica (L.) Vig. was long other Linnaean and Linnaean-linked herbaria. considered to be the only European representative of the The specimen in UPS-BURSER has precedence in genus. -
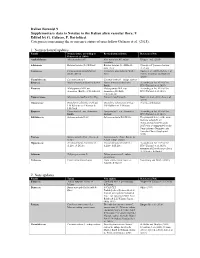
9 Edited by G. Galasso, F. Bartolucci Categories Concerning the Occurrence Status of Taxa Follow Galasso Et Al
Italian Botanist 9 Supplementary data to Notulae to the Italian alien vascular flora: 9 Edited by G. Galasso, F. Bartolucci Categories concerning the occurrence status of taxa follow Galasso et al. (2018). 1. Nomenclatural updates Family Nomenclature according to Revised nomenclature References/Note Galasso et al. (2018) Asphodelaceae Aloë maculata All. Aloë maculata All. subsp. Klopper et al. (2020) maculata Solanaceae Batatas batatas (L.) H.Karst. Batatas batatas (L.) H.Karst., Synonym of Ipomoea batatas nom. inval. (L.) Lam. Lauraceae Cinnamomum glanduliferum Camphora glandulifera (Wall.) Huang et al. (2016), Rohde et al. (Wall.) Meisn. Nees (2017), Trofimov and Rohwer (2020) Cucurbitaceae Cucumis sativus L. Cucumis sativus L. subsp. sativus Rosaceae Malus domestica (Borkh.) Borkh. Malus domestica (Suckow) According to Art. 41.4 of the Borkh. ICN (Turland et al. 2018) Rosaceae Malus pumila Mill. var. Malus pumila Mill. var. According to Art. 41.4 of the domestica (Borkh.) C.K.Schneid. domestica (Suckow) ICN (Turland et al. 2018) C.K.Schneid. Papaveraceae Meconopsis cambrica (L.) Vig. Papaver cambricum L. Kadereit et al. (2011), Liu et al. (2014) Onagraceae Oenothera oakesiana (A.Gray) Oenothera oakesiana (A.Gray) Priority combination J.W.Robbins ex S.Watson & J.W.Robbins ex S.Watson J.M.Coult. Rosaceae Pyrus malus L. var. domestica Pyrus malus L. var. domestica According to Art. 41.4 of the Borkh. Suckow ICN (Turland et al. 2018) Salviniaceae Salvinia adnata Desv. Salvinia molesta D.S.Mitch. The proposal to reject the name Salvinia adnata Desv. (Schwartsburd and Miranda 2017) was recommended by the Nomenclature Committee for Vascular Plants (Applequist 2019) Poaceae Setaria pumila (Poir.) Roem. -

Chemo-Dahlgrenogram of the Tribe Papavereae
Chemo-Dahlgrenogram of the Tribe Papavereae P. Tétényi Research Institute of Medicinal Plants P.O. Box 11 2011 Budakalász Hungary Keywords: chemotaxonomy, Romneya, Cathcartia, Meconopsis, Stylomecon, Roemeria Abstract The review puts forward and extends the chemo-dahlgrenogram of the genus Papaver over Papavereae. — Tertiary Cathcartia owns watery latex as Romneya (Pa- laeoaster’s sister), but is evoluted by sole sand-like crystals in seeds’ coats. On the other hand, Cathcartia, that synthesizes indenobenzoazepine alkaloids, differs sub- stantially from Meconopsis. Therefore Cathcartia is an independent entity represent- ing the starting-point for Papavereae. — Meconopsis cambrica Vig. is missing Me- conopsis constituents mentioned before, but synthesizes armepavine derivative aporphines, as well as meconic acid and nudicaulin (stated at Papaver sections, but being unproved at Meconopsis taxa). Therefore the species belongs to Papaver genus, and its original name Papaver cambricum Linnaeus is effective again. — Meconopsis polycarpic (sometimes monocarpic) species of ser. Simplicifoliae accumulate retro- protoberberines, but ser. Grandes only berberine. Monocarpic species of Aculeatae and Robustae are characterized by isopavines, orientaline derivative aporphines and harmane alkaloids. Monocarpic life span of Meconopsis taxa is analogous with the biennial Papaver sect. Meconidium. — Separation of the Californian, annual Sty- lomecon heterophylla with phthalideisoquinoline alkaloid content from the Asian ge- nus Meconopsis is also proved, alike to some annual or biennial Papaver taxa. — Roemeria species with aporphines and protoberberines are joined to Papaver section Argemonidium. Chemism of Roemeria refracta with rhoeadanes, promorphinanes is more similar to Papaver genus, in contrast to R. hybrida synthesizing spirocyclic- coupled proaporphines, unique at Papavereae. — The previous Papaver chemo- dahlgrenogram must be changed by detaching sect. -
The Vascular Plants of Devon
The vascular plants of Devon Flora as Lycopodium inundatum L. p.754, Atlas p.1 (6), 1987 Lycopodiaceae onwards (3). VC3 l Huperzia selago (L.) Bernh. ex Shrank & Mart. SX55 Edges of pools in old china clay workings at Fir Clubmoss – Map 1 Smallhanger Waste, Crownhill Down, SX576595, Occasional. Native. A Boreo-arctic Montane species, on the 1980, R.Davies (Wigston et al. 1981); in three places, edge of its range in Devon. Mainly restricted to Dartmoor, locally dominant over several square metres where it has been recorded on 31 tors since 2000, amongst small Molinia caerulea tussocks at principally on ledges and amongst clitter, with a few SX578596, 1994, R.E.N.Smith ; 30 colonies varying substantial populations in wet heath by small streams. from a few plants to an almost continuous patch Individual plants are occasionally found on heath or measuring 32 x 40 m in two clay pits and by two blanket peat remote from any of the bigger populations. In ponds, SX5759, 2000, N.F.Stewart (Stewart & the past it has been recorded from Exmoor and various FitzGerald 2001); in northwest corner of an sites in East and South Devon, but there is only one recent abandoned china clay pit, near the shallowly record from East Devon, at Hense Moor. flooded floor, several hundred, perhaps a few Flora as Lycopodium selago L. p.753, Atlas p.1 (38), 1987 thousand plants, SX576597, 2003, P.D.Pullen . Three onwards (35). tiny plants in the bottom of ditch on Headon Down, Map 1. Huperzia selago (Fir Clubmoss) Lutton, SX585595, 2007, A.J.Byfield. -
Fjölrit Náttúrufræðistofnunar Annotated Checklist of Vascular Plants Iceland
FJÖLRIT NÁTTÚRUFRÆÐISTOFNUNAR ANNOTATED CHECKLIST OF VASCULAR PLANTS ICELAND ANNOTATED CHECKLIST OF VASCULAR PLANTS OF ICELAND Paweł Wąsowicz 57 57 FJÖLRIT NÁTTÚRUFRÆÐISTOFNUNAR ANNOTATED CHECKLIST OF VASCULAR PLANTS OF ICELAND Paweł Wąsowicz 57 FJÖLRIT NÁTTÚRUFRÆÐISTOFNUNAR Nr. 57, mars 2020 Fjölrit Náttúrufræðistofnunar er ritröð sem hóf göngu sína árið 1985. Birtar eru greinar og skýrslur eftir starfsmenn og fræðimenn sem vinna í samvinnu við þá. Í hverju hefti er ein sjálfstæð grein um náttúrufræði. Útgáfan er óregluleg. Greinar eru ritaðar á íslensku með enskum útdrætti. Þær mega einnig vera á ensku en þá skal ávallt fylgja ítarlegur útdráttur á íslensku. Vitnið til þessa rits á eftirfarandi hátt – Recommended citation: Paweł Wąsowicz 2020. Annotated checklist of vascular plants of Iceland. Fjölrit Náttúrufræðistofnunar nr. 57. Garðabær: Náttúrufræðistofnun Íslands. DOI: 10.33112/1027-832X.57 Ritnefnd María Harðardóttir, Guðmundur Guðmundsson og Guðríður Gyða Eyjólfsdóttir Kápumynd Holtasóley (Dryas octopetala) Stílfærð teikning Anette Theresia Meier eftir teikningu Carl Axel Magnus Lindman úr bókinni Bilder ur Nordens Flora Umbrot María Harðardóttir Útgefandi NÁTTÚRUFRÆÐISTOFNUN ÍSLANDS Urriðaholtsstræti 6–8 210 Garðabæ Sími: 590 0500 Netfang: [email protected] www.ni.is Prentun Prentmet Oddi ©Náttúrufræðistofnun Íslands 2020 ISSN 1027-832X ANNOTATED CHECKLIST OF VASCULAR PLANTS OF ICELAND Paweł Wąsowicz EFNISYFIRLIT ABSTRACT 5 INTRODUCTION 7 DEFINITIONS OF TERMS AND ABBREVIATIONS USED 10 CHECKLIST OF VASCULAR PLANTS – PLÖNTUTAL 11 ÚTDRÁTTUR Á ÍSLENSKU 167 REFERENCES – HEIMILDIR 169 INDEX OF GENERA – SKRÁ YFIR LATNESK ÆTTKVÍSLAHEITI 174 INDEX OF ICELANDIC NAMES – SKRÁ YFIR TEGUNDAHEITI 180 FJÖLRIT NÁTTÚRUFRÆÐISTOFNUNAR 192 – 3 – FJÖLRIT 57 NÁTTÚRUFRÆÐISTOFNUN ÍSLANDS, mars 2020 ANNOTATED CHECKLIST OF VASCULAR PLANTS OF ICELAND Paweł Wąsowicz ABSTRACT The present edition of the annotated checklist is a non-native of unknown age and 19 taxa qualified as comprehensive catalogue of all vascular plant taxa: archaeophytes. -

Floral Humidity in Flowering Plants: a Preliminary Survey
Harrap, M. J. M., Hempel de Ibarra, N., Knowles, H. D., Whitney, H. M., & Rands, S. A. (2020). Floral humidity in flowering plants: a preliminary survey. Frontiers in Plant Science, 11, [249]. https://doi.org/10.3389/fpls.2020.00249 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.3389/fpls.2020.00249 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Frontiers Media at https://www.frontiersin.org/articles/10.3389/fpls.2020.00249/full . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ fpls-11-00249 March 5, 2020 Time: 20:7 # 1 ORIGINAL RESEARCH published: 06 March 2020 doi: 10.3389/fpls.2020.00249 Floral Humidity in Flowering Plants: A Preliminary Survey Michael J. M. Harrap1*, Natalie Hempel de Ibarra2, Henry D. Knowles1, Heather M. Whitney1 and Sean A. Rands1 1 School of Biological Sciences, University of Bristol, Bristol, United Kingdom, 2 Centre for Research in Animal Behaviour, School of Psychology, University of Exeter, Exeter, United Kingdom The area of space immediately around the floral display is likely to have an increased level of humidity relative to the environment around it, due to both nectar evaporation and floral transpiration. -

The Powdery Mildews of Wales
The Powdery Mildews (Erysiphales) of Wales: Llwydni Blodeuoogg (Eryypsiphales) Cymru: Arthur O. Chater & Ray G. Woods Summary The powdery mildew fungi form a well circumscribed group of parasitic fungi in the Order Erysiphales within the Phylum Ascomycetes (the “spore shooters”). If the host plant can be accurately identified the task of identifying the powdery mildew is relatively easy. Presented here is a catalogue of host plant species and their powdery mildews which have been reported from Wales or which might occur in Wales, with a synopsis of characters to enable a fungus to be identified where more than one occurs on a particular host. Over 700 taxa of powdery mildews are known world-wide with over 166 reported from Britain. Catalogued here by the Vice-counties within Wales in which they occur, are over 122 taxa of powdery mildews. Representatives of all five Tribes of the powdery mildews occur in Wales. As many of the wild host plants diminish in extent, the fungi that are dependent on them grow scarcer. This guide, we hope, will stimulate their study and enable conservation priorities to be established. Crynodeb Ffurfir y ffwng llwydni blodeuog grwp cyfyngiedig o ffyngau parasitig o fewn yr Urdd Erysiphales sydd o fewn y Ffylwm Ascomycetes (y ‘saethwyr sborau’). Os yw planhigion cynhaliol yn cael eu enwi’n gywir, mae’r dasg o enwi y llwydni blodeuog yn weddol hawdd. Wedi ei gyflwyno yma mae catalog o rywogaethau o blanhigion cynhaliol a’u llwydni blodeuol sydd wedi eu cofnodi yng Nghymru neu efallai yn bodoli yng Nghymru, gyda chrynodeb o nodweddion sy’n galluogi i’r ffwng gael ei enwi’n gywir yn yr achosion ble mae mwy nac un yn bodoli ar blanhigyn cynhaliol arbennig. -

Papaver – Mohn-Arten in Nordrhein-Westfalen
Jahrb. Bochumer Bot. Ver. 7 237–266 2016 Papaver – Mohn-Arten in Nordrhein-Westfalen F. WOLFGANG BOMBLE & ARMIN JAGEL 1 Einleitung In Nordrhein-Westfalen sind fünf Mohn-Arten heimisch, neben dem bekannten Klatsch-Mohn (Papaver rhoeas ) und dem Sand-Mohn ( Papaver argemone ) noch drei Arten aus der Arten- gruppe des Saat-Mohns ( Papaver dubium agg.). In der Geländeliste der Kartierung der Flora Nordrhein-Westfalens in den Jahren 1989–1998 waren aus letztgenanntem Aggregat aller- dings nur zwei aufgeführt, der Saat-Mohn i. e. S. (Papaver dubium s. str.) und der Gelb- milchende Mohn ( Papaver lecoqii ). Da der Verkannte Mohn ( Papaver confine ) damals auch in der Florenliste NRW fehlte bzw. dort nur in einer Anmerkung genannt war (RAABE & al. 1995: 95), blieb er bei der Kartierung weitgehend unbeachtet. Obwohl G. H. LOOS bereits 1995 (JAGEL & LOOS 1995) auf die Häufigkeit dieser Art hinwies und anmerkte, dass sie zumindest in Mittelwestfalen sogar häufiger sei als Papaver dubium s. str., konnte der Kartie- rungsrückstand in NRW nicht mehr aufgeholt werden und es entstand eine Verbreitungs- karte, die den Wissensstand der Kartierer, nicht aber die Verbreitung der beiden Kleinarten darstellte (vgl. HAEUPLER & al. 2003). Auch heute werden die beiden Arten noch nicht konsequent getrennt, auch weil die Unterscheidung nicht immer einfach ist. Die dritte Art des Saat-Mohn-Aggregats, P. lecoqii, wird meist lediglich anhand des farbigen Milchsaftes unter- schieden. Dieses Pflanzenporträt soll insbesondere dazu dienen, diese drei Kleinarten anhand von zahlreichen Fotos darzustellen und das Erkennen zu erleichtern. Miteinbezogen werden außerdem weitere Papaver -Arten, die in NRW neophytisch auftreten. Schon länger wird der Bastard-Mohn ( Papaver hybridum ) in Nordrhein-Westfalen adventiv beobachtet. -

An Open Vegetation Seed Bank Community at Worsley in Salford (V.C.59), Revealed During Construction of the New Royal Horticultural Society Garden at Bridgewater
British & Irish Botany 2(4): 377-406, 2020 Galeopsis speciosa (Lamiaceae): an Open Vegetation seed bank community at Worsley in Salford (v.c.59), revealed during construction of the new Royal Horticultural Society Garden at Bridgewater Michael J. Crawley Imperial College London, Silwood Park, Ascot, SL5 7PY Corresponding author: [email protected] This pdf constitutes the Version of Record published on 7th December 2020 Abstract This paper provides a baseline flora for the site of the new garden of the Royal Horticultural Society at Worsley New Hall in Salford (v.c.59). During construction, 35,000 m3 of top-soil, sub-soil and spoil were stripped and stored onsite; species recruiting from these seed banks were monitored 2017-2020, leading to the description of a new Galeopsis speciosa Open Vegetation plant community. Four commercial wildflower mixes were used during post-construction landscaping in 2019, and their establishment was assessed in 2020. It will be interesting to follow the survival of these introduced species, many of which are not native to the site. Keywords: landscaping; seed bank; RHS; introduced species; establishment; wildflower seed mix Introduction Francis Egerton (1736-1803), 3rd Duke of Bridgewater, made a fortune in the mid- 18th century from coal mines at Worsley in Lancashire. Known as a pioneer of canal construction, he is regarded as the father of British inland navigation. He commissioned the Bridgewater Canal to service the coal mines, the first true canal in the modern world, which was built for him by his agent John Gilbert with advice from the engineer James Brindley. -

Cranmore Catalogue
NORTHERN IRELAND HERITAGE GARDENS TRUST OCCASIONAL PAPER, no. 8 (2016) A listing of plants cultivated between 1807 and 1825 by John Templeton (1766–1825) at Cranmore, Malone, Belfast E. Charles Nelson "Thoughtful, observant, enquiring, he reasoned out horticultural techniques for himself from what he knew about Ireland's native plants." Born in Belfast in 1766, John Templeton began to take an interest in gardening when he was about 20 years old "and soon made his flower-garden an object of attention". In 1793, according to his biographer, Templeton laid out an experimental garden in what was formerly an orchard and osiery. A stream was redirected to pass through the new garden; this probably formed the serpentine pond that survived long after John Templeton's death. A rock-garden was created. Into this place Templeton brought "from various parts of the world, rare and useful plants, which he endeavoured to naturalize in this climate". That is the nub of his pioneering horticulture. 1 He had his own methods for germinating seeds. "I never apply artificial heat", he wrote, "In sowing seeds I think great numbers are lost by sowing too deep". So he sowed his seeds on the surface of the soil: "Nature sows on the surface and leaves to chance the covering of them by leaves or Moss". Following this principle, John put a thick wad of chopped moss over freshly sown seeds; he knew that this would keep the soil in the pots moist even in summer ''all the watering that is necessary is just to keep the [soil] dark color'd" – while in winter the moss-blanket "prevented the plants from being affected with frost".