FULL ISSUE Vol 56, No 4; Oct.- Dec., 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2016-17
Jeee|<ekeâ efjheesš& Annual Report 201201666---20120120177 केb6ीय ितबती अaययन िव िवcालय Central University of Tibetan Studies (Deemed University) Sarnath, Varanasi - 221007 www.cuts.ac.in Conference on Buddhist Pramana A Glance of Cultural Programme Contents Chapters Page Nos. 1. A Brief Profile of the University 3 2. Faculties and Academic Departments 9 3. Research Departments 45 4. Shantarakshita Library 64 5. Administration 79 6. Activities 89 Appendices 1. List of Convocations held and Honoris Causa Degrees Conferred on Eminent Persons by CUTS 103 2. List of Members of the CUTS Society 105 3. List of Members of the Board of Governors 107 4. List of Members of the Academic Council 109 5. List of Members of the Finance Committee 112 6. List of Members of the Planning and Monitoring Board 113 7. List of Members of the Publication Committee 114 Editorial Committee Chairman: Dr. Dharma Dutt Chaturvedi Associate Professor, Dean, Faculty of Shabdavidya, Department of Sanskrit, Department of Classical and Modern Languages Members: Shri R. K. Mishra Documentation Officer Shantarakshita Library Shri Tenzin Kunsel P. R. O. V.C. Office Member Secretary: Shri M.L. Singh Sr. Clerk (Admn. Section-I) [2] A BRIEF PROFILE OF THE UNIVERSITY 1. A BRIEF PROFILE OF THE UNIVERSITY The Central University of Tibetan Studies (CUTS) at Sarnath is one of its kind in the country. The University was established in 1967. The idea of the University was mooted in course of a dialogue between Pandit Jawaharlal Nehru, the first Prime Minister of India and His Holiness the Dalai Lama with a view to educating the young Tibetan in exile and those from the Himalayan regions of India, who have religion, culture and language in common with Tibet. -

53Rd AIIMS ANNUAL REPORT 2008–2009
53rd AIIMS ANNUAL REPORT 2008–2009 All India Institute of Medical Sciences New Delhi 110029 Edited jointly by: Dr Sunil Chumber, Additional Professor, Department of Surgical Disciplines and Sub-Dean (Academic) Dr Tanuj Dada, Associate Professor, Dr R.P. Centre for Ophthalmic Sciences Dr Venkata Karthikeyan C, Assistant Professor, Department of Otorhinolaryngology (ENT) Dr S.K. Maulik, Professor, Department of Pharmacology Dr Raj D. Mehra, Professor, Department of Anatomy Dr Kameshwar Prasad, Professor, Department of Neurology Dr S. Rastogi, Professor, Department of Orthopaedics Dr Sushma Sagar, Assistant Professor, JPNA Trauma Centre Dr Peush Sahni, Professor, Department of Gastrointestinal Surgery Dr Pratap Sharan, Professor, Department of Psychiatry Dr D.N. Sharma, Assistant Professor, Dr BRA, Institute Rotary Cancer Hospital Dr Subrata Sinha, Professor and Head, Department of Biochemistry Dr Sanjay Kumar Sood, Assistant Professor, Department of Physiology Dr Sachin Talwar, Assistant Professor, Department of C.T.V.S. February 2010 Printed at Saurabh Printers Pvt. Ltd., A-16, Sector-IV, NOIDA (U.P.) All India Institute of Medical Sciences The All India Institute of Medical Sciences (AIIMS) was established in 1956 as an institution of national importance by an Act of Parliament with the objects to develop patterns of teaching in undergraduate and postgraduate medical education in all its branches so as to demonstrate a high standard of medical education to all medical colleges and other allied institutions in India; to bring together in one place educational facilities of the highest order for the training of personnel in all important branches of health activity and to attain self-sufficiency in postgraduate medical education. -

List of Fellows (Name-Wise) Upto 2016
LIST OF FELLOWS (NAME-WISE) UPTO 2016 0. Description Year 1. Abdul Kalam, A.P.J. Biomedical Engineering July 1995 DMIT. Former President, Republic of India. Res: 10 Rajaji Marg, New Delhi-110001. Permanent Address: No. 2, Mosque Street, Rameswaram, Ramanathapuram District, Tamil Nadu-623526. Tel: Off: (011) 3015321, 3014930, Res: (04567) 6493708, Fax: 2300756, E-mail: [email protected] (b 1931) (d.2015) Gen. Amir Chand Oration (NAMS, 1997-98) Padma Bhushan (1981); Padma Vibhushan (1990); Bharat Ratna (1997); D.Sc (h.c.) from several Universities; National Design Award; Dr. Biren Roy Space Award; Om Prakash Bhasin Award; National Nehru Award by Govt. of Madhya Pradesh; GM Modi Award for Science 1996; HK Firodia Award for Excellence in S&T 1996; Veer Savarkar Award 1998; Hon Fellow-Institution of Electronics and Telecommunication Engineers. 2. Abraham, Jacob Neurosurgery 1984 MS, MS (Neuro), FACS, FACA. Res: 10, 15th Avenue, Harrington Road, Chennai- 600031. Tel: Res: (044) 28363211, 42849258, Mobile: 09940118382, E-mail: [email protected] (b.1931). Basanti Devi Amir Chand Prize (ICMR, 1984); Sachs Memorial Lecturer, USA (1989). 3. Achari, Kamala Obstetrics and Gynecology 1982 MS, FRCOG, FICS, FACS. Emeritus Professor, Patna Medical College, Patna-800001 (Bihar). Res: 'Tirumalai', 21/D Road No.10, Rajendra Nagar, Patna- 800016. (b.1924) (d. 2014). 4. Adithan, C. Pharmacology July 2003 MD, PhD, FIMSA, FIPS. Former Professor & Head, Department of Pharmacology, Jawaharlal Institute of Postgraduate Medical Education & Research, Pondicherry- 605006. Currently: Director-CIDRF and Professor of Pharmacology, Mahatma Gandhi Medical College and Research Institute, Pondicherry-607403. Res: Flat No. 1, Srinivas Towers, Vazhudavour Road, Kathirkamam, Pondicherry-605009. -

Download Arogya Setu App for Those Living in Hotspot Areas of the State
Monthly Current AffairsTitle Capsule & Quiz MayTitle 2020 The countries placed on the list by the Trump administration on intellectual property (IP) related issues are Algeria, Argentina, Chile, China, India, Indonesia, Russia, Saudi Arabia, Ukraine and Venezuela. According to the World Intellectual Property International Labour Day: 1 May Right Organization, the Intellectual Property is the creation of literary and artistic works, symbols, names, images. World Tuna Day: 02 May Labour Day or International Workers' Day is observed each year on the first day of May to celebrate achievements of the working class. The day, also called 'May Day', is also observed as a public holiday in many countries. On 1 May, 1886, labour unions in the US decided to go on a strike demanding that World Tuna Day is observed on May 2 every year. workers should not be made to work for more than 8 hours a day. The United Nations (UN) established the Just three days after the strike began, a blast World Tuna Day to raise awareness about the occurred in Chicago's Haymarket Square tuna fish and its importance to humans and leaving many dead. earth. To honor those who died in the blast, the It is also observed to promote more International Socialist Conference declared 1 sustainable fishing practices. May as a day designated for labourers. The United Nations general assembly officially voted in favour of observing World US places India on priority watch list for Tuna Day in the year 2016. intellectual property protection World Tuna Day was observed for the first time in the year 2017. -

Alphabetical List of Recommendations Received for Padma Awards - 2014
Alphabetical List of recommendations received for Padma Awards - 2014 Sl. No. Name Recommending Authority 1. Shri Manoj Tibrewal Aakash Shri Sriprakash Jaiswal, Minister of Coal, Govt. of India. 2. Dr. (Smt.) Durga Pathak Aarti 1.Dr. Raman Singh, Chief Minister, Govt. of Chhattisgarh. 2.Shri Madhusudan Yadav, MP, Lok Sabha. 3.Shri Motilal Vora, MP, Rajya Sabha. 4.Shri Nand Kumar Saay, MP, Rajya Sabha. 5.Shri Nirmal Kumar Richhariya, Raipur, Chhattisgarh. 6.Shri N.K. Richarya, Chhattisgarh. 3. Dr. Naheed Abidi Dr. Karan Singh, MP, Rajya Sabha & Padma Vibhushan awardee. 4. Dr. Thomas Abraham Shri Inder Singh, Chairman, Global Organization of People Indian Origin, USA. 5. Dr. Yash Pal Abrol Prof. M.S. Swaminathan, Padma Vibhushan awardee. 6. Shri S.K. Acharigi Self 7. Dr. Subrat Kumar Acharya Padma Award Committee. 8. Shri Achintya Kumar Acharya Self 9. Dr. Hariram Acharya Government of Rajasthan. 10. Guru Shashadhar Acharya Ministry of Culture, Govt. of India. 11. Shri Somnath Adhikary Self 12. Dr. Sunkara Venkata Adinarayana Rao Shri Ganta Srinivasa Rao, Minister for Infrastructure & Investments, Ports, Airporst & Natural Gas, Govt. of Andhra Pradesh. 13. Prof. S.H. Advani Dr. S.K. Rana, Consultant Cardiologist & Physician, Kolkata. 14. Shri Vikas Agarwal Self 15. Prof. Amar Agarwal Shri M. Anandan, MP, Lok Sabha. 16. Shri Apoorv Agarwal 1.Shri Praveen Singh Aron, MP, Lok Sabha. 2.Dr. Arun Kumar Saxena, MLA, Uttar Pradesh. 17. Shri Uttam Prakash Agarwal Dr. Deepak K. Tempe, Dean, Maulana Azad Medical College. 18. Dr. Shekhar Agarwal 1.Dr. Ashok Kumar Walia, Minister of Health & Family Welfare, Higher Education & TTE, Skill Mission/Labour, Irrigation & Floods Control, Govt. -

NEFT Details: : Bombay Chamber of Commerce and Industry Bank
JOURNALISM OF COURAGE SINCE 1932 Dear friends & fellow members, Government/Municipal Hospitals, a lot of members have come forward. the below mentioned Government/Municipal Hospitals on one hand & their empanelled vendors on the other to ensure that the right & much needed equipment & consumables were delivered to these hospitals. List of hospitals facilitated by the Chamber: Hospital Type Description (* in process) Cost/Value B.Y.L. Nair Hospital Consumable *3 Ply Surgical Mask `12.8 lakhs PPE kits Equipment *ICU Beds JJ Hospital Consumable 3 Ply Surgical Mask & Face Shields `17.1 lakhs Sanitizer Equipment Mobile Digital X-ray Kasturba Hospital Consumable 3 Ply Surgical Mask & Face Shields `15.4 lakhs Sanitizer Equipment Auto Bio Chemistry Analyzer *Coagulometer KEM Hospital Consumable PPE kits `2.8 lakhs Sion Hospital Consumable PPE kits `2.8 lakhs NMMC Vashi Hospital Consumable 3 Ply Surgical Mask & Face Shields `10.9 lakhs PPE kits Sanitizer Equipment *Video Laryngoscope Total ` Lakhs (approx.) `61.8 lakhs As the COVID-19 cases are only increasing, we would once again request our esteemed members to continue contributing in every possible way. NEFT Details: : Bombay Chamber of Commerce and Industry Bank Name : State Bank of India Bank Address : Horniman Circle, Mumbai Main Branch, Mumbai Samachar Marg,Fort Account Type : Current Account Account No. : 10996680930 IFSC Code : SBIN0000300 In order to continue serving our members, Bombay Chamber has been regularly uploading its website with information on Government/other Regulatory Authorities on COVID-19, including relating to employment. On behalf of the Bombay Chamber team, I wish you and your families a stay safe during the lock-in. -

English Version
NATIONAL ACADEMY OF MEDICAL SCIENCES (INDIA) ANNUAL REPORT 2019-2020 NAMS House Ansari Nagar, Mahatma Gandhi Marg, New Delhi - 110029 i Postal Address: NATIONAL ACADEMY OF MEDICAL SCIENCES (INDIA) NAMS House, Ansari Nagar, Mahatma Gandhi Marg, New Delhi – 110029 Telephone: Academy : 011-26588718 President : 011-26588792 Secretary : 011-26589289 Fax No.: 011-26588992 E-mail: [email protected] Website: http://nams-india.in ii iii iv CONTENTS Page No. The Council ─ 2019-20 1 Officers & Executive Staff 2 Editorial Board of Annals of NAMS 3-4 A. Organizational Activities 5-62 Organisational Activities & Annual Meeting 7 Award of Fellowships & Memberships at the Annual Meeting 8-19 Lifetime Achievement Award 20 Orations and Awards 21-25 NAMS Amritsar Award 26 Meetings of the Council 27 Election of Fellows – 2019 27-31 Election of Members – 2019 31-42 Candidates proposed for Membership (MNAMS) on passing DNB Examination 43-48 Fellows/Members on rolls of the Academy 48 Nominations of Medical Scientists for Orations and Awards – 2019-20 49-52 Lifetime Achievement Award for the year 2019 53 Maintenance of Building 53 Publication of Annals 5 4 Obituary 55 Text of the address by the President Dr. Saroj Chooramani Gopal, delivered at the Annual Convocation on 12th October 2019, at Bhopal (Annexure I) 56-60 Text of the address by the Chief Guest His Excellency Governor of Madhya Pradesh Shri Lal Ji Tandon, at the Annual Convocation on 12th October 2019, at Bhopal (Annexure II) 61-62 B. Academic Report 63 Continuing Medical Education Programme 65 Report of activities April 1, 2019-March 31, 2020 65-70 State-wise distribution of Extramural CME Programmes 71 Extramural CME Programmes of NAMS Chapters (Annexure III) 72-73 v Report on Extramural CME Programmes (Annexure IV) 74-95 The Medical Scientists’ Exchange Programmes (Health Manpower Development) 96 Report on Intramural CME Programmes (Annexure V) 97-103 NAMS Chapters (Annexure VI) 104-107 Emeritus Professors of NAMS 108 Report of Academic Committee NAMS Website 110 C. -

Download Download
Annals of the National Academy of Medical Sciences (India) EN JUB D IL L E Volume 53, No. 2 O E G April-June, 2017 ISSN 0379 - 038X (Print) ISSN 2454-5635 (Online) Annals of the National Academy of Medical Sciences (India) -: A quarterly Journal :- Editor Dr. Sanjeev Misra Associate Editors Dr. V Mohan Kumar Dr. Kuldeep Singh Assistant Editor Dr. Mohan Kameswaran Editorial Board Editorial Associates Dr. Snehalata Deshmukh Dr. M V Padma Srivastava Dr. W Selvamurthy Dr. R K Chadda Dr. J N Pande Dr. Deep N Srivastava Dr. Prema Ramachandran Dr. Promila Bajaj Dr. H S Sandhu Dr. N R Jagannathan Dr. Lalita S Kothari Dr. Subrata Sinha Dr. Vinod Paul Dr. Ravinder Goswami Dr. Sanjay Wadhwa Dr. (Brig) Velu Nair Members of the Advisory Board Dr. P K Misra Dr. Rajeshwar Dayal Dr. M Berry Dr. C S Saimbi Air Marshal Dr. M S Boparai Dr. R Madan Dr. Y K Chawla Dr. Raj Kumar Dr. P K Dave Dr. Mukund S Joshi Dr. Amod Gupta Dr. Kamal Buckshee Dr. Ravi Kant Dr. Haribhai L Patel Dr. Balram Airan Dr. I C Verma Dr. Saroj Chooramani Gopal Dr. Geeta K Vemuganti Emeritus Editor: Prof. J.S. Bajaj Annual Subscription Rates Inland Rs. 500.00 Foreign $ 30.00 £ 15.00 Single Copy Rs. 150.00 Correspondence All correspondence concerning the Journal should be addressed to: Honorary Secretary National Academy of Medical Sciences (India) NAMS House, Ansari Nagar, Mahatma Gandhi Marg, New Delhi-110029 Tel.: 011-26589289 Email: [email protected] Website: www.nams-india.in Ann Natl Acad Med Sci (India), 53(2): 66-120, 2017 ANAMS 53(2): 66-120, 2017 CONTENTS Editorial i Sanjeev Misra, Kuldeep Singh Circulating Antimicrobial Peptide LL-37 Status in Type 1 Diabetes Mellitus and 66 its Relation with Glycemic Control Himika Chawla, Parmita Kar, Soma Saha, Urvashi B. -

High Court of Delhi Advance Cause List
HIGH COURT OF DELHI ADVANCE CAUSE LIST LIST OF BUSINESS FOR ND FRIDAY, THE 22 SEPTEMBER, 2017 INDEX PAGES 1. APPELLATE JURISDICTION 01 TO 41 2. SPECIAL BENCH (APPLT. SIDE) 42 TO 54 3. COMPANY JURISDICTION 55 TO 68 4. ORIGINAL JURISDICTION 69 TO 80 5. REGISTRAR GENERAL/ 81 TO 93 REGISTRAR(ORGL.)/ REGISTRAR (ADMN.)/ JOINT REGISTRARS(ORGL). 22.09.2017 1 (APPELLATE JURISDICTION) 22.09.2017 [Note : Unless otherwise specified, before all appellate side courts, fresh matters shown in the supplementary lists will be taken up first.] COURT NO. 1 (DIVISION BENCH-I) HON'BLE THE ACTING CHIEF JUSTICE HON'BLE MR. JUSTICE C. HARI SHANKAR AFTER NOTICE MISC. MATTERS ____________________________ 1. FAO(OS) 29/2017 M/S KONKA GROUP COMPANY LTD LEX INFINI CM APPL. 3587/2017 Vs. M/S A2VP DISTRIBUTORS & WITH FAO(OS) 66/2017 ANR 2. FAO(OS) 66/2017 M/S A2VP DISTRIBUTORS PVT LTD NIMANNIYU SHARMA & ANR Vs. M/S KONKA GROUP COMPANY LTD 3. W.P.(C) 1960/2014 BAWANA FACTORY WELFARE YUDHVIR SINGH CHAUHAN,HEMANT ASSOCIATION (REGD.) GUPTA,PRATIMA GUPTA,AJAY Vs. GOVT. OF NCT OF DELHI & ARORA,VIVEK GOYAL,H C ORS BHATIA,AVNISH AHLAWAT,PRABHSAHAY KAUR,SATYAKAM,ANSUYA SALWAN 4. W.P.(C) 9498/2015 RAMESH KUMAR & ORS. SAMEER MENDIRATTA & CM APPL. 32334/2017 Vs. DELHI DEVELOPMENT ASSOCIATES,SUSHIL DUTT AUTHORITY & ORS. SALWAN,ARJUN MITRA,YEESHU JAIN,AJAY ARORA,SOVI BIPNEET SINGH,SK TRIPATHI 5. W.P.(C) 4552/2016 SANJAY TIWARI VIVEK Vs. GOVT. OF NCT OF DELHI & NARAYAN,SATYAKAM,KIRTIMAN ORS SINGH 6. W.P.(C) 11276/2016 PEOPLES ALL INDIA ANTI ATTORNEYS AT LAW,NAMRATA CORRUPTION HUKIM,SWATY SINGH MALIK Vs. -

Sarnath, Varanasi - 221007
Jeee|<ekeâ efjheesš& Annual Report 201201777---20120120188 केb6ीय उTच ितबती िशMा संथान Central Institute of Higher Tibetan Studies (Deemed University) Sarnath, Varanasi - 221007 www.cuts.ac.in Dignitaries and audience during Golden Jubilee Celebration His Holiness on the occasion of 92 nd AIU Meet His Holiness releasing the souvenir of Golden Jubilee Celebration Contents Chapters Page Nos. 1. A Brief Profile of the Institute 3 2. Faculties and Academic Departments 9 3. Research Departments 43 4. Shantarakshita Library 63 5. Administration 77 6. Activities 87 Appendices 1. List of Convocations held and Honoris Causa Degrees Conferred on Eminent Persons by CIHTS 104 2. List of Members of the CIHTS Society 106 3. List of Members of the Board of Governors 108 4. List of Members of the Academic Council 110 5. List of Members of the Finance Committee 113 6. List of Members of the Planning and Monitoring Board 114 7. List of Members of the Publication Committee 115 Editorial Committee Chairman: Prof. Dharma Dutt Chaturvedi Professor, Dean, Faculty of Shabdavidya, Head, Department of Sanskrit, Head, Department of Classical and Modern Languages Members: Dr. Lobsang Dorjee Research Assistant, Dept. of Restoration Dr. Devi Prasad Singh Professional Assistant, Shantarakshita Library Dr. Jasmeet Gill Guest Faculty, English, Dept. of Classical & Modern Languages Member Secretary: Shri M.L. Singh Sr. Clerk, (Admn. Section-I) [2] A BRIEF PROFILE OF THE INSTITUTE 1. A BRIEF PROFILE OF THE INSTITUTE The Central Institute of Higher Tibetan Studies (CIHTS) at Sarnath is one of its kind in the country. The University was established in 1967. -
(Ms) Membership No: Res: Mobile: Email
SUPREME COURT BAR ASSOCIATION Name & Address Name & Address 1 Wangmo,Rinchen (Ms) 5 A. Roopashri (Mrs.) Membership no: W-00060 Membership no: A-00121 Res: H.No-84, Block-B, Sector A-9, Pocket-1,Narela, Res: 3/2, St. Johns Road,, Shivan Chetty Garden Post, Delhi 110040 Bangalore 560042 Mobile: 8826232720 Tel: 080-25549660 Email: [email protected] Off: 3/2, St. Johns Road,, Shivan Chetty Garden Post, Bangalore 560042 Tel: 080-25307104 2 A. Hanumantha Reddy Mobile: 9886012342 Email: [email protected] Membership no: A-00103 Res: Plot no.28, Vivekananda Enclave, Road No.2, 6 A.Mohan Doss Banjara Hills, Hyderabad, AP 500034 Tel: 040-23744322 Membership no: A-00094 Mobile: 09849536633 Email: [email protected] Res: No-13/2, Khan Street, Choolaimedu, Chennai 600094 Tel: 044-23741149 3 A. Kalamegam V. Arumugam Ch: 71, Law Chamber, Madras High Court, Chennai 600094 Membership no: A-00090 Tel: 044-23741149 Mobile: 09884043335 Res: Old No-3, New No-7, Mosque Street, Chepauk, Channai 600005 Tel: 044-28552939 7 A.S Naushad Ch: 153, Additional Law Charmber, Madras High Court, Madras Membership no: A-00175 Mobile: 9840062370 Email: [email protected] Res: Ishel Near.B.H.S, Attingal, Trivandrum, Kerala 695101 Off: Flat No77, Tower No-13, 4th Floor,, Supreme 4 A. Raja Enlclave, M.V. Ph-1, Delhi Mobile: 9847130707 A-00811 Membership no: Email: [email protected] Res: A-33, Gulmohar Park, New Delhi 110049 Tel: 26531222-333 8 A.S. Rao Mobile: 9013180381,9999864553 Membership no: A-00575 Email: [email protected] Res: H.No.27, 3rd floor, Street No.7,, Pratap Nagar, Mayur Vihar Phase-I,, New Delhi Tel: 011-22756891 Ch: 411, M.C. -
List of Candidates Called for Computer Typing Test
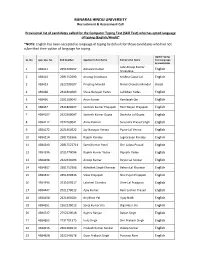
BANARAS HINDU UNIVERSITY Recruitment & Assessment Cell Provisional list of candidates called for the Computer Typing Test (Skill Test) who has opted language of typing (English/Hindi)* *NOTE: English has been accepted as language of typing by default for those candidates who had not submitted their option of language for typing. Opted Typing SL. No. App. Seq. No. Roll Number Applicant’s Full Name Father's Full Name Test Language by candidates Late Anoop Kumar 1 438411 2651400167 Ashwani Kumar English Srivastava 2 438414 2581752690 Anurag Srivastava Krishna Gopal Lal English 3 438423 2622290107 Pradeep Mandal Nimai Chandra Mandal Hindi 4 438428 2341800683 Shiva Narayan Yadav Lal Bihari Yadav English 5 438456 2101380043 Arun Kumar Ramlayak Giri English 6 438467 2341800207 Santosh Kumar Prajapati Ram Nayan Prajapati English 7 4384107 2622500087 Santosh Kumar Gupta Bachcha Lal Gupta English 8 4384112 2072260041 Annu Kumari Surendra Prasad Singh English 9 4384172 2021450322 Jay Narayan Verma Pyare Lal Verma English 10 4384214 2581751666 Rajesh Pandey Jugnarayan Pandey English 11 4384240 25817522711 Sanoj Kumar Patel Shri Lalata Prasad English 12 4384296 2021470096 Rajesh Kumar Yadav Rajnath Yadav English 13 4384298 2622430196 Anoop Kumar Birjoo Lal Sonkar English 14 4384307 2581752968 Abhishek Singh Kharwar Behari Lal Kharwar English 15 4384347 2651400346 Vikas Prajapati Shiv Pujan Prajapati English 16 4384396 2511600217 Lakshmi Chandra Gore Lal Prajapati English 17 4384447 2921270012 Ajay Kumar Ram Lochan Prasad English 18 4384458 2621460184 Brij Bhan Pal Vijay Nath English 19 4384651 2262230013 Saroj Kumar Jha Digambar Jha English 20 4384727 2752220018 Rajeev Ranjan Baban Singh English 21 4384863 2731701375 Indu Singh Shri Prakash Singh English 22 4384916 2661480119 Prakash Kumar Sonkar Dileep Sonkar English 23 4384928 2622140178 Gyan Prakash Singh Punwasi Ram English Opted Typing SL.