Mortality Rates for Juvenile Pink Oncorhynchus Gorbuscha and Chum O
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Phylogeny of Oncorhynchus (Euteleostei: Salmonidae) Based on Behavioral and Life History Characters
Copeia, 2007(3), pp. 520–533 The Phylogeny of Oncorhynchus (Euteleostei: Salmonidae) Based on Behavioral and Life History Characters MANU ESTEVE AND DEBORAH A. MCLENNAN There is no consensus between morphological and molecular data concerning the relationships within the Pacific basin salmon and trout clade Oncorhynchus. In this paper we add another source of characters to the discussion. Phylogenetic analysis of 39 behavioral and life history traits produced one tree structured (O. clarki (O. mykiss (O. masou (O. kisutch (O. tshawytscha (O. nerka (O. keta, O. gorbuscha))))))). This topology is congruent with the phylogeny based upon Bayesian analysis of all available nuclear and mitochondrial gene sequences, with the exception of two nodes: behavior supports the morphological data in breaking the sister-group relationship between O. mykiss and O. clarki, and between O. kisutch and O. tshawytscha. The behavioral traits agreed with molecular rather than morphological data in placing O. keta as the sister-group of O. gorbuscha. The behavioral traits also resolve the molecular-based ambiguity concerning the placement of O. masou, placing it as sister to the rest of the Pacific basin salmon. Behavioral plus morphological data placed Salmo, not Salvelinus, as more closely related to Oncorhynchus, but that placement was only weakly supported and awaits collection of missing data from enigmatic species such as the lake trout, Salvelinus namaycush. Overall, the phenotypic characters helped resolve ambiguities that may have been created by molecular introgression, while the molecular traits helped resolve ambiguities introduced by phenotypic homoplasy. It seems reasonable therefore, that systematists can best respond to the escalating biodiversity crisis by forming research groups to gather behavioral and ecological information while specimens are being collected for morphological and molecular analysis. -

History of Lahontan Cutthroat Trout in Spring Creek, Utah
Spring Creek Population History of the Pyramid Lake Rediscovery (Again) Unfortunately, given its small size, the trout Lahontan Cutthroat population at Spring Creek has a very low In October 2009, a team from Weber State probability of survival. It lacks the numbers The Lahontan cutthroat trout, Oncorhynchus University in conjunction with personnel and space necessary to maintain sufficient clarkii henshawi, is native to the Lahontan Basin from the DWR identified several specimens genetic diversity. It is believed that for a on the border between California and Nevada. believed to be of a pure or hybrid strain of mountain stream cutthroat population to For thousands of years it thrived and played the Pyramid Lake Lahontan cutthroat trout survive it must have a minimum of 3.3 km an important economic and cultural role in Spring Creek in Uintah, Utah. Using of habitat and an abundance in the area of among the Native American tribes of the electrofishers and dip nets, a 600 m stretch 0.3 fish per meter.3 Based on our region. The largest strain of this fish of the stream was sampled. A maximum observations, the Spring Creek population originated in Pyramid Lake, in western of 16 different individuals was collected in A Unique Environment has a maximum abundance of 0.1 fish/m Nevada and has reached recorded weights of two sampling trips. The fish appeared to Spring Creek’s unique vegetation and only 200 m of habitat. However, against up to 41 pounds, making it the largest “The Fish that Won’t Die” be restricted to a 200 m stretch. -
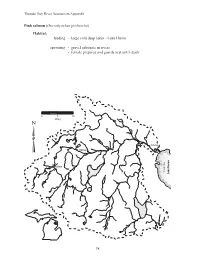
Lake Huron Spawning
Thunder Bay River Assessment Appendix Pink salmon (Oncorhynchus gorbuscha) Habitat: feeding - large cold deep lakes - Lake Huron spawning - gravel substrate in rivers - female prepares and guards nest until death 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 98 Thunder Bay River Assessment Appendix Coho salmon (Oncorhynchus kisutch) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cold streams, later into pools spawning - cold streams and rivers - swifter water of shallow gravelly substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 99 Thunder Bay River Assessment Appendix Rainbow trout (Oncorhynchus mykiss) Habitat: feeding - cold clear water of rivers and Lake Huron - moderate current spawning - gravelly riffles above a pool - smaller tributaries 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 100 Thunder Bay River Assessment Appendix Chinook salmon (Oncorhynchus tshawyscha) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cool streams, later into pools spawning - gravelly substrate in cool streams 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 101 Thunder Bay River Assessment Appendix Round whitefish (Prosopium cylindraceum) Habitat: feeding - lakes, rivers, and streams spawning - shallows of lakes and rivers - gravel or rock substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 102 Thunder Bay River Assessment Appendix Atlantic salmon (Salmo salar) Habitat: feeding - young: gravel substrate streams - adults: Lake Huron -

Salmon Fact Sheet
THE WILD SALMON SEAFOOD MARKET’S GUIDE TO W I L D P A C I F I C S A L M O N Salmon Pacific Salmon occur from northern California along the Pacific Coast throughout the Pacific Ocean, Bering Sea and Arctic Ocean waters adjacent to Alaska. Salmon are anadromous, that is, they spawn in fresh water and the young migrate to the sea where they mature. The mature Salmon returns to the stream of their birth to spawn. Nutrition Few single foods bring as many valuable contributions to the table as Salmon. An excellent source of high-quality protein, containing all the essential amino acids. The fats in Salmon are predominately unsaturated. These fats are evidenced to reduce the risk of heart disease. Availability Although each species has a particular season, small fisheries of wild salmon occur periodically, making fresh salmon (often hard to find and expensive) available throughout the year. Your best values will come during peak salmon season, May through September. Frozen salmon (often frozen at sea) is available during the off season. Also known as Chinook Salmon. Also known as Silver Salmon. Highly desired for King The largest of the species and the most Coho both table use and smoking. Coho salmon offers prominent of the salmon known for its high oil firm meat with excellent flavor slightly milder than content and distinctive, rich flavor. King and Sockeye. Average size from 5 to 40 lbs. Average size from 4 - 9 lbs. Available May - September Available June - September Copper River & Yukon River King Also known as Chum Salmon. -

Coastal Cutthroat Trout (Oncorhynchus Clarkii Clarkii) Data
Coastal Cutthroat Trout (Oncorhynchus clarkii clarkii) Data: 1999 NOAA Status Review; 2007 PSMFC Review Partners: AK, BC, CA, OR , WA, FWS, USFS, USGS, NMFS, PSMFC, Tribes._____________ Species Status review: evidence that current smolt counts represent a fragment of historic counts. In addition, we are Coastal cutthroat trout (Oncorhynchus clarkii certain about the disappearance of many clarkii) have been considered a vulnerable populations that were once fished in certain indicator species in recent years and have been highly developed ecoregions. petitioned for listing under the Endangered Species Act (ESA). In 1996, National Marine CCT are the only sub-species of O. clarkii Fisheries Service (NMFS) listed the Umpqua without a multi-agency management plan in River coastal cutthroat trout (CCT) as a place. In 2006, in an effort to remedy this threatened species under the Endangered situation and as part of the decision to withdraw Species Act of 1973, as amended (16 U.S.C. the listing of the SWWC-ESU, Pacific States 1531 et seq). Following this listing, NMFS Marine Fisheries Commission (PSMFC) and conducted a status review of the species FSW initiated a voluntary effort among state, throughout their distributional range in the tribal, federal and provincial agencies that lower 48 state region of North America. represent agencies throughout the distributional During that process NMFS identified six range of CCT. The goal of this effort is to Evolutionary Significant Units (ESU’s). The coordinate agency efforts, share knowledge Fish and Wildlife Service (FWS) and NMFS (meta data approach), and advance our have, in the past, jointly managed CCT under understanding of CCT with the long-term goal of the ESA and on April 5, 1999, the agencies developing a consistent framework for the published a joint proposal to list the management, research, restoration, and southwestern Washington-Columbia River conservation of the subspecies. -

Imagine the Silver Beauty and the Fighting Spirit of Atlantic Salmon; The
Sakhalin Silver Text and Photos: Clemens Ratschan Imagine the silver beauty and the fighting spirit of Atlantic salmon; the complex, unpredictable life- history of sea trout and combine with the ferocious take and body mass of a predatory taimen. This will give you a glimpse of what fishing for Sakhalin taimen, the silver of the Russian Far East, is about. AM PLEASED TO introduce Siberian taimen, Hucho taimen. No this fish to the readers of wonder, scientists also erroneously Chasing Silver, because in related this far-eastern species to many respects it forms a the large-sized, non-anadromous missing link between the predators of the genus Hucho, which Ifishery for anadromous salmon and is a branch of the salmonoid tree for huchen, a big predatory non- that occurs exclusively in Eurasia. anadromous salmonoid in my home In Central Europe, Hucho hucho is country of Austria (‘Danube salmon’ restricted to the Danube System, in English. See article “Taimen” by where self-sustaining stocks are Wolfgang Hauer, issue 3/2010). presently only found in a handful of Sakhalin taimen is one of the rivers in Germany, Austria, Slovakia least-known salmonid species among and former Yugoslavia. Huchen is non-Russian fishermen; even many very closely related to the already- Russians tend to confuse it with the mentioned Siberian taimen. The latter | 62 | Chasing Silver Fly Fishing Magazine April’s Fav Five www.chasingsilvermagazine.com | 63 | Sakhalin Silver inhabits a distant, vast range from a habits. But one ecological feature expeditions to Japan. Later, the fish few places in European Russia to the is unique – all members of the true was assigned to the genus Parahucho, Lena and Amur rivers in the very far huchen live exclusively in fresh water, with regard to some obvious east of northern Asia. -

Species Fact Sheet Coastal Cutthroat Trout Oncorhynchus Clarkii
Species Fact Sheet Coastal Cutthroat Trout Oncorhynchus clarkii STATUS: SPECIES OF The Southwestern Washington/Lower Columbia CONCERN River Distinct Population Southwestern Segment of Coastal cutthroat Washington/Lower trout potentially occurs in these Washington counties: Thurston, Columbia River Distinct Lewis, Yakima, Mason, Pacific, Population Segment Grays Harbor, Wahkiakum, Cowlitz, Clark, Skaminia, Klickitat, (Map may reflect historical as well as recent sightings) In 1999, the southwestern Washington/lower Columbia River Distinct Population Segment of coastal cutthroat trout, Oncorhynchus clarkii clarkii, was listed as threatened by National Marine Fisheries Service and the U.S. Fish and Wildlife Service FR 64(64): 16397-414. Subsequently, the Fish and Wildlife Service assumed sole regulatory jurisdiction. Based on changes in forest management regulation, the latest information indicating better than expected total populations in a large portion of the area, and an improved understanding of the ability of freshwater forms to produce anadromous progeny, the Fish and Wildlife Service withdrew the listing proposal in 2002. Current and Historical Status This Distinct Population Segment (DPS) includes populations in the Columbia River and its tributaries downstream from the Klickitat River in Washington and Fifteenmile Creek in Oregon to the Columbia River estuary; and the Willamette River and its tributaries downstream from Willamette Falls, to its confluence with the Columbia River, as well as in tributaries of Gray's Harbor and Willapa Bay. The southwestern Washington-lower Columbia River region historically supported highly productive coastal cutthroat trout populations. Coastal cutthroat trout are well distributed in most river basins in this geographic region, although probably in lower numbers relative to historical population sizes. -

Full Text in Pdf Format
Vol. 27: 277–287, 2015 ENDANGERED SPECIES RESEARCH Published online May 13 doi: 10.3354/esr00675 Endang Species Res OPENPEN ACCESSCCESS Causes of the drastic loss of genetic variation in the Critically Endangered Formosa landlocked salmon of Taiwan Te-Hua Hsu1, Keisuke Takata2, Hiroshi Onozato3, Jin-Chywan Gwo1,* 1Department of Aquaculture, National Taiwan Ocean University, Keelung 20224, Taiwan 2Faculty of Science, Shinshu University, Matsumoto-city, Nagano 390-8621, Japan 3Matsumoto Institute of Microorganisms Co. Ltd., Matsumoto-city, Nagano 390-1241, Japan ABSTRACT: The use of hatchery-reared fish to replenish existing threatened wild populations has been shown to reduce or change the natural genetic diversity of the wild populations. In this study, the genetic diversity of wild Formosa landlocked salmon Oncorhynchus formosanus in its main habitat of the Chichiawan Stream in Taiwan was examined after a large-scale escape of hatchery- cultivated fish. Approximately 3000 individuals (the descendants of only 5 pairs of wild salmon) es- caped from an old hatchery when Typhoon Ariel breached the hatchery in the fall of 2004. The ge- netic diversity of the wild population was extremely low at that time, and declined further between 2004 and 2008 following the escape of hatchery fish. We hypothesize that the decline in genetic di- versity of the wild population was mainly caused by a population bottleneck in 2005, and that ge- netic homogeneity since 2005 was caused by breeding of the escaped hatchery fish (which showed low genetic diversity) that survived the floods of 2004. This supports the possibility that the drastic decline in genetic diversity between 2004 and 2008 was caused by the genetic effects of the escaped hatchery fish, and demonstrates the risk of introducing hatchery fish into the wild. -

Oncorhynchus Nerka) in Nearshore Waters of the Kodiak Archipelago
OCS Study MMS 2001-059 Final Report Feeding Ecology of Maturing Sockeye Salmon (Oncorhynchus nerka) in Nearshore Waters of the Kodiak Archipelago by Albert V. Tyler1 Charles O. Swanton2 Bruce C. McIntosh1 Principal Investigators 1 School of Fisheries and Ocean Sciences University of Alaska Fairbanks Fairbanks, AK 99775-7220 2 Alaska Department of Fish and Game 1300 College Road Fairbanks, AK 99701-1599 E-mail:[email protected] [email protected] [email protected] August 2001 Table of Contents List of Tables ................................................................................................................................ iv List of Figures ............................................................................................................................... v Abstract ......................................................................................................................................... 1 Introduction ................................................................................................................................... 1 Objectives ...................................................................................................................................... 4 Methods ......................................................................................................................................... 4 Incidence of feeding .................................................................................................................. 6 Diet .......................................................................................................................................... -

Rainbow Trout (Oncorhynchus Mykiss)
Rainbow Trout (Oncorhynchus mykiss) General Information Like the brown trout, rainbow trout are a non-native salmonid species and are distributed annually throughout the state through the FW's trout stocking program. These stockings, however, have not resulted in wide spread reproducing populations as seen with brown trout. The number of reproducing populations within the state is minimal. Native Pacific drainages from Northwestern Mexico to the Kushowin River in Alaska. In Canada, Range found in the Peace and Athabasca Rivers in the Mackenzie drainage (Smith 1985). Habitat Description River: Clear, cold stream systems with a 1:1 pool – riffle ration with areas of slow deep water; abundant in- stream cover and stable water flow, Base flow > 50% of average annual daily flow is considered excellent, 25 – 50% of annual daily flow is only considered fair. (Raleigh, 1984). Lake: Clear, cold, deep lakes, typically oligotrophic. Require tributary streams with a gravel substrate to reproduce (Raleigh, 1984). Optimum Habitat Requirements Diet Dissolved Oxygen > 7 mg/l Fry Insects Temperature 12 – 19°C Juveniles Aquatic and terrestrial insects pH 6.5 – 8.0 Adults Fish, aquatic and terrestrial insects Turbidity 0 – 30 JTU’s Notes: Opportunistic feeders Current Reproduction Time of Year February – March Age Males Mature 2 - 3 Temperature Range 10 – 15.5°C Age Females Mature 3 Water Depth Nest Built by female Gravel, size dependent Substrate Egg Type Demersal on size of individual Time of Day Day / Night Parental Care None 28 – 40 (temp. Critical pH Days to Hatching dependent) Velocity Range Oxygen Level Notes: Almost exclusively stream spawners; streams with no inlet or outlet generally do not have a reproducing population of rainbow trout. -

Salmo Salar, Rainbow Trout Oncorhynchus Mykiss, Cod Gadus
DISEASES OF AQUATIC ORGANISMS Published January 27 Vol. 18: 37-44, 1994 Dis. aquat. Org. l Disposition of 14~-sarafloxacinin Atlantic salmon Salmo salar, rainbow trout Oncorhynchus mykiss, cod Gadus morhua and turbot Scophthalmus maximus, as demonstrated by means of whole-body autoradiography and liquid scintillation counting Bernt Martinsen, Tor Einar Horsberg, Kristian Ingebrigtsen, Inger Lise Gross Department of Pharmacology. Microbiology and Food Hygiene, Division of Pharmacology and Toxicology. Norwegian College of Veterinary Medicine, PO Box 8146 Dep., N-0033 Oslo, Norway ABSTRACT The absorption, distribution and elimination of I4C-labelled sarafloxacin hydrochloride were studied by means of whole-body autoradiography and liquid scintillation counting. The drug was administered to Atlantic salmon Salmo salar, rainbow trout Oncorhynchus mykiss, cod Gadus morhua and turbot Scophthalmus maximus, either intravenously or orally in a single dose of 9.6 (3.57) and 9.7 (3.44)mg kg-' (MBq kg-') respectively. The Atlantic salmon, rainbow trout and cod were held in seawater at a temperature of 7.9 f 0.2"C, and the turbot at 12.1 + l.l°C. In the intravenously dosed groups, the drug was rapidly distributed to all major tissues and organs except the central nervous system. After the distribution phase, the levels of radioactivity were higher in most organs and tissues than in blood The most noticeable differences between the species were the lower levels of radio- activity in the Liver, and the higher levels in the muscle tissue, of cod compared to the salmonids. lncomplete absorption was observed following oral administration of sarafloxacin to Atlantic salmon. -

I Diseases of Aquatic Organisms
DISEASES OF AQUATIC ORGANISMS Vol. 11: 93-97, l991 Published August 8 Dis. aquat. Org. I l Infectivity of a rickettsia isolated from coho salmon Oncorhynchus kisutch ' Veterinary Sciences Faculty, University of Chile, Santiago, Chile Association of Chilean Salmon Farmers, Puerto Montt, Chile Laboratory for Fish Disease Research, Department of Microbiology, Oregon State University, Hatfield Marine Science Center, Newport, 0regon 973656296, USA Department of Microbiology, Oregon State University, Nash Hall 220, Corvallis, Oregon 97331-3804, USA ABSTRACT: Two species of salmonids were tested for their susceptibility to infection by a rickettsia isolated in cell culture from diseased coho salmon Oncorhynchus kisutch in Chile. Mortality approached 100 % in coho and Atlantic salmon Salmo salar injected with 10-fold dilutions of cell culture medium containing the rickettsia. Typical disease signs were present in the coho salmon but were not observed in inoculated Atlantic salmon. However, the rickettsia was recovered in pure culture from moribund fish in each of the injected groups of either species. The rickettsia was thereby demonstrated to be pathogenic and the cause of the ongoing epizootic affecting salmonids cultured in Chile. Horizontal transmission was not demonstrated in a group of uninoculated coho salmon held in the same tank with experimentally infected fish. INTRODUCTION seen. Mild lesions in pancreatic, cardiac, and ovarian tissues may also be present (Cvitanich et al. 1990). Salmonid aquaculture is a rapidly growing industry In 1989, as part of an extensive effort to determine in the sheltered coastal waters of southern Chile; how- the cause of the continuing epizootic, kidney tissue ever, coho salmon Oncorhynchus kisutch reared in from moribund coho salmon was inoculated onto a seawater netpens in this area have experienced an chinook salmon embryo cell line (CHSE-214) (Lannan ongoing epizootic first documented by Bravo & Cam- et al.