Supplementary Material
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
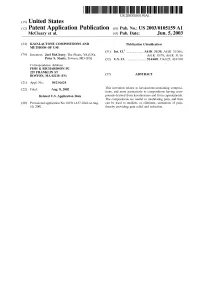
(12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 Mccleary Et Al
US 200301 05159A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 McCleary et al. (43) Pub. Date: Jun. 5, 2003 (54) KAVALACTONE COMPOSITIONS AND Publication Classification METHODS OF USE (51) Int. Cl." ....................... A61K 31/35; A61K 31/366; (76) Inventors: Joel McCleary, The Plains, VA (US); A61K 35/78; A61K 31/16 Peter S. Staats, Towson, MD (US) (52) U.S. Cl. ........................... 514/460; 514/625; 424/760 Correspondence Address: FISH & RICHARDSON PC 225 FRANKLIN ST BOSTON, MA 02110 (US) (57) ABSTRACT (21) Appl. No.: 10/214,624 (22) Filed: Aug. 8, 2002 This invention relates tO kavalactone-containing composi tions, and more particularly to compositions having com Related U.S. Application Data pounds derived from kavalactones and from capsaicinoids. The compositions are useful in modulating pain, and thus (60) Provisional application No. 60/311,437, filed on Aug. can be used to mediate, or eliminate, Sensations of pain, 10, 2001. thereby providing pain relief and reduction. US 2003/0105159 A1 Jun. 5, 2003 KAVALACTONE COMPOSITIONS AND METHODS 0006. In one embodiment, the invention relates to an OF USE analgesic topical composition having: (a) a kavalactone; (b) capsaicinoid or Synthetic derivatives thereof; and (c) a CROSS-REFERENCE TO RELATED pharmaceutically acceptable carrier; wherein the weight APPLICATIONS ratio of(a):(b) is from 5000:1 to 1:2 (e.g., 800:1 to 1:1; 500:1 to 5:1). In other aspects, the composition includes an effec 0001. This application claims benefit of U.S. application tive amount of kavalactones, active kavalactones, or capsai Ser. -
Effects of Mimosine Administered to a Perfused Area of Skin in Angora Goats by R
Downloaded from British Journal of Nutrition (1996), 15, 69-19 69 https://www.cambridge.org/core Effects of mimosine administered to a perfused area of skin in Angora goats BY R. PUCHALA, S. G. PIERZYNOWSKI, T. SAHLU* AND S. P. HART E. (Kika) de la Garza Institute for Goat Research, Langston University, Langston, Oklahoma 73050, USA . IP address: (Received I1 November 1994 - Revised 15 March 1995 -Accepted I1 May 1995) 170.106.34.90 The effect of mimosine on a perfused area of skin tissue was studied using an isolated perfusion technique. Four mature Angora wethers (body weight 35 (SE 2.3) kg) were cannulated bilaterally with indwelling silicone catheters in the superficial branches of the deep circumflex iliac artery and vein. Mimosine , on (40 mg/kg metabolic weight (Wo75)per d) was infused intra-arterially into one iliac artery of each goat for 3 d and saline was infused in the contralateral (control) iliac artery. Iliac venous blood samples were 27 Sep 2021 at 22:01:16 taken from both sides along with arterial samples from the carotid artery. Mimosine infusion elevated plasma mimosine in the carotid artery (52.6 (SEM19.21) pol/l) and iliac vein on the saline-treated side to 54.1 (SEM 16-31)~ol/l and in the iliac vein on the mimosine-treated side to 191.3 (SEM1914) pmol/l (P < 0.01). Mimosine decreased feed intake (2.3 v. 0.6 kg/d, ~~~0.29;P < 0.001) and water consumption (5-2 v. 1.3 litres/d, SEM 0.67; P < 0.001). -

Review on the Nutritive Value and Toxic Aspects of Leucaena Leucocephala
Trop Anim Prod 1979 4:2 113 A REVIEW ON THE NUTRITIVE VALUE AND TOXIC ASPECTS OF LEUCAENA LEUCOCEPHALA U ter Meulen1, S Struck1, E Schulke2 and E A El Harith1 1 Institut fur Tierphysiologie und Tierernahrung der Universitat Gottingen Oskar-Kellner-Weg 6, Weende 3400 Gottingen, West Germany This review discusses the nutritive value of Leucaena leucocephala and its mimosine toxicity when used as a forage for livestock. Chemical analysis and feeding trial. have indicated that Leucaena leaf-meal with its high protein, calcium, -carotene and xanthophyll contents, is potentially a valuable feed for livestock in the tropics. The symptoms of mimosine toxicity in cattle, sheep, poultry, goats horses, pigs and rats ire discussed, The chemical value of mimosine and the possible mechanism of its toxicity are reviewed. Possible solutions to the toxicity problem are presented together with the suggestion that further studies be initiated to overcome this problem. Key Words: Leucaena, mimosine toxicity, livestock, forage, feeding trials The uses of the tropical legume Leucaena leucocephala are quite versatile. These uses include its function as a source of firewood and timber,its role in soil erosion control (Dijkman 1950), its ability to provide shade for other plants as well as its function in maintaining the fertility of the soil and of serving as a nutritious forage for animal feed (Ruskln 1977). Presently the greatest use of this plant in animal nutrition is its incorporation in cattle feed. Leucaena leaf-meal, with its rich protein, minerals and vitamin content, is also becoming a popular ingredient in poultry feeds in the tropics (D'Mello and Taplin 1978). -

Bufadienolides from the Skin Secretions of the Neotropical Toad Rhinella Alata (Anura: Bufonidae): Antiprotozoal Activity Against Trypanosoma Cruzi
molecules Article Bufadienolides from the Skin Secretions of the Neotropical Toad Rhinella alata (Anura: Bufonidae): Antiprotozoal Activity against Trypanosoma cruzi Candelario Rodriguez 1,2,3 , Roberto Ibáñez 4 , Luis Mojica 5, Michelle Ng 6, Carmenza Spadafora 6 , Armando A. Durant-Archibold 1,3,* and Marcelino Gutiérrez 1,* 1 Centro de Biodiversidad y Descubrimiento de Drogas, Instituto de Investigaciones Científicas y Servicios de Alta Tecnología (INDICASAT AIP), Apartado 0843-01103, Panama; [email protected] 2 Department of Biotechnology, Acharya Nagarjuna University, Nagarjuna Nagar, Guntur 522510, India 3 Departamento de Bioquímica, Facultad de Ciencias Naturales, Exactas y Tecnología, Universidad de Panamá, Apartado 0824-03366, Panama 4 Smithsonian Tropical Research Institute (STRI), Balboa, Ancon P.O. Box 0843-03092, Panama; [email protected] 5 Centro Nacional de Metrología de Panamá (CENAMEP AIP), Apartado 0843-01353, Panama; [email protected] 6 Centro de Biología Celular y Molecular de Enfermedades, INDICASAT AIP, Apartado 0843-01103, Panama; [email protected] (M.N.); [email protected] (C.S.) * Correspondence: [email protected] (A.A.D.-A.); [email protected] (M.G.) Abstract: Toads in the family Bufonidae contain bufadienolides in their venom, which are charac- Citation: Rodriguez, C.; Ibáñez, R.; terized by their chemical diversity and high pharmacological potential. American trypanosomiasis Mojica, L.; Ng, M.; Spadafora, C.; is a neglected disease that affects an estimated 8 million people in tropical and subtropical coun- Durant-Archibold, A.A.; Gutiérrez, M. tries. In this research, we investigated the chemical composition and antitrypanosomal activity Bufadienolides from the Skin of toad venom from Rhinella alata collected in Panama. -

Preventive and Therapeutic Effects of Chinese Herbal Compounds Against Hepatocellular Carcinoma
molecules Review Preventive and Therapeutic Effects of Chinese Herbal Compounds against Hepatocellular Carcinoma Bing Hu 1,*, Hong-Mei An 2, Shuang-Shuang Wang 1, Jin-Jun Chen 3 and Ling Xu 1 1 Department of Oncology and Institute of Traditional Chinese Medicine in Oncology, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China; [email protected] (S.-S.W.); [email protected] (L.X.) 2 Department of Science & Technology, Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 202032, China; [email protected] 3 Department of Plastic & Reconstructive Surgery, Shanghai Key Laboratory of Tissue Engineering, The Ninth People’s Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai 200011, China; [email protected] * Correspondence: [email protected]; Tel.: +86-21-64385700 Academic Editor: Derek J. McPhee Received: 16 November 2015 ; Accepted: 20 January 2016 ; Published: 27 January 2016 Abstract: Traditional Chinese Medicines, unique biomedical and pharmaceutical resources, have been widely used for hepatocellular carcinoma (HCC) prevention and treatment. Accumulated Chinese herb-derived compounds with significant anti-cancer effects against HCC have been identified. Chinese herbal compounds are effective in preventing carcinogenesis, inhibiting cell proliferation, arresting cell cycle, inducing apoptosis, autophagy, cell senescence and anoikis, inhibiting epithelial-mesenchymal transition, metastasis and angiogenesis, regulating immune function, reversing drug -

New Markers in the Mycotox Profile
New Markers in the MycoTOX Profile We are happy to announce the addition of four new mycotoxin markers to our MycoTOX Profile. The test now includes 11 mycotoxins from 40 species of mold, making it by far the most comprehensive and competitively priced mycotoxin test available. It also still more sensitive and accurate than other tests available, because we use LC/MS/MS technology. Here is an overview of the four new mycotoxin markers: Gliotoxin Gliotoxin (GTX) is produced by the mold genus Aspergillus. Aspergillus spreads in the environment by releasing conidia which are capable of infiltrating the small alveolar airways of individuals. In order to evade the body’s defenses Aspergillus releases Gliotoxin to inhibit the immune system. One of the targets of Gliotoxin is PtdIns (3,4,5) P3. This results in the downregulation of phagocytic immune defense, which can lead to the exacerbation of polymicrobial infections. Gliotoxin impairs the activation of T-cells and induces apoptosis in monocytes and in monocyte-derived dendritic cells. These impairments can lead to multiple neurological syndromes. Mycophenolic Acid Mycophenolic Acid (MPA) produced by the Penicillium fungus. MPA is an immunosuppressant which inhibits the proliferation of B and T lymphocytes. MPA exposure can increase the risk of opportunistic infections such as Clostridia and Candida. MPA is associated with miscarriage and congenital malformations when the woman is exposed in pregnancy. Dihydrocitrinone Dihydrocitrinone is a metabolite of Citrinin (CTN), which is a mycotoxin that is produced by the mold species Aspergillus, Penicillium, and Monascus. CTN exposure can lead to nephropathy, because of its ability to increase permeability of mitochondrial membranes in the kidneys. -

Fungal Keratitis: Immune Recognition, Neutrophil-Hyphae Interactions, And
FUNGAL KERATITIS: IMMUNE RECOGNITION, NEUTROPHIL-HYPHAE INTERACTIONS, AND FUNGAL ANTI-OXIDATIVE DEFENSES by SIXTO MANUEL LEAL JR. Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Thesis Advisor: Eric Pearlman, Ph.D. Department of Pathology CASE WESTERN RESERVE UNIVERSITY August, 2012 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the dissertation of ______________________________________________________ candidate for the Ph.D. degree *. (signed)_______________________________________________ (chair of the committee) ________________________________________________ ________________________________________________ ________________________________________________ ________________________________________________ ________________________________________________ (date) _______________________ *We also certify that written approval has been obtained for any proprietary material contained therein. Dedication I dedicate this cumulative work to the invisible hand that has blessed my personal and academic life with incredible people, guidance, talent, courage, perseverance, and productivity. 3 Table of Contents List of Figures 7 List of Tables 9 Acknowledgements 10 List of Abbreviations 12 Abstract 14 Chapter 1. Introduction Fungi in their natural environment 16 Fungi and human disease 18 Fungi that cause human corneal infection 21 Fungal keratitis- Clinical characteristics and outcome 22 Anti-microbial Defenses at the Ocular Surface 23 Immune Recognition of Fungi 27 β2 integrins -

Comparative Pharmacokinetic Study of Luteolin After Oral Administration Of
Vol. 8(16), pp. 422-428, 29 April, 2014 DOI 10.5897/AJPP2013.3835 ISSN 1996-0816 African Journal of Pharmacy and Copyright © 2014 Author(s) retain the copyright of this article Pharmacology http://www.academicjournals.org/AJPP Full Length Research Paper Comparative pharmacokinetic study of luteolin after oral administration of Chinese herb compound prescription JiMaiTong in spontaneous hypertensive rats (SHR) and Sprague Dawley (SD) rats Zhao-Huan Lou1, Su-Hong Chen2, Gui-Yuan Lv1*,Bo-Hou Xia1, Mei-Qiu Yan1, Zhi-Ru Zhang1 and Jian-Li Gao1 1Institute of Material Medica, Zhejiang Chinese Medical University, 548 Binwen Road, Hangzhou, 310053, China. 2Academy of Tradition Chinese Medicine, Wenzhou Medical University, Wenzhou 325035, China. Received 7 August, 2013; Accepted 15 April, 2014 JiMaiTong (JMT), a Chinese herb compound prescription consisted of Flos chrysanthemi Indici, Spica prunellae and Semen cassiae for anti-hypertension. Luteolin is one of the major bioactivity compositions in F. chrysanthemi Indici in JMT. There are some reports about pharmacokinetics of luteolin in extract of F. chrysanthemi and husks of peanut in normal rats, but it lacked pharmacokinetic information of luteolin residing in a Chinese herb compound prescription in hypertensive animal models. The present study aimed to develop a high-performance liquid chromatography with photodiode array detection (HPLC-DAD) method for determination of luteolin in rat plasma and for pharmacokinetic study after oral administration of JMT to spontaneous hypertensive rats (SHR) and normal Sprague Dawley (SD) rats. After oral administration of JMT to SHR and SD rats, respectively the content of luteolin in blood samples at different time points were determined by a reversed-phase high- performance liquid chromatography (RP-HPLC) coupled with liquid-liquid phase extraction. -

Antidepressant-Like Behavioral and Neurochemical Effects of the Citrus
Available online at www.sciencedirect.com Life Sciences 82 (2008) 741–751 www.elsevier.com/locate/lifescie Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin ⁎ Li-Tao Yi, Jian-Mei Li, Yu-Cheng Li, Ying Pan, Qun Xu, Ling-Dong Kong State Key Laboratory of Pharmaceutical Biotechnology, School of Life Sciences, Nanjing University, Nanjing 210093, PR China Received 14 July 2007; accepted 16 January 2008 Abstract Apigenin is one type of bioflavonoid widely found in citrus fruits, which possesses a variety of pharmacological actions on the central nervous system. A previous study showed that acute intraperitoneal administration of apigenin had antidepressant-like effects in the forced swimming test (FST) in ddY mice. To better understand its pharmacological activity, we investigated the behavioral effects of chronic oral apigenin treatment in the FST in male ICR mice and male Wistar rats exposed to chronic mild stress (CMS). The effects of apigenin on central monoaminergic neurotransmitter systems, the hypothalamic–pituitary–adrenal (HPA) axis and platelet adenylyl cyclase activity were simultaneously examined in the CMS rats. Apigenin reduced immobility time in the mouse FST and reversed CMS-induced decrease in sucrose intake of rats. Apigenin also attenuated CMS-induced alterations in serotonin (5-HT), its metabolite 5-hydroxyindoleacetic acid (5-HIAA), dopamine (DA) levels and 5-HIAA/ 5-HT ratio in distinct rat brain regions. Moreover, apigenin reversed CMS-induced elevation in serum corticosterone concentrations and reduction in platelet adenylyl cyclase activity in rats. These results suggest that the antidepressant-like actions of oral apigenin treatment could be related to a combination of multiple biochemical effects, and might help to elucidate its mechanisms of action that are involved in normalization of stress- induced changes in brain monoamine levels, the HPA axis, and the platelet adenylyl cyclase activity. -

615.954Foo3rded.Pdf
Index Acquired immunodeficiency syndrome pinnipeds (seals, sea lions, walruses), 47ll-1 (AIDS), 451-3, 474, 475 second intermediate hosts, 471 Acromelic acids, 605 Arcobacterspp., 272-3 Acute non-bacterial gastroenteritis see Arizona spp., 344 Noroviruses Ascaris suum, 476 Adenoviruses,404 Aspergillusflavus, A. parasiticus see Aeromonas spp., 342-3 Aflatoxin Aeramonas hydrophile, 342-3 Aspergillus mycotoxins (nitropropionic acid, Aflatoxin, 586--9, 609-12 territrems, sterigmatocystin), 597--8 Aspergillusjlavus, A. parasiticus, 586 Astroviruses, 402-3 biosynthesis, 587-8 carcinogenesis in humans, 588-9 Bacillary dysentery, 359-60 ebselen, 625 Bacillus cereus gastroenteritis, 563-77, hepatitis B virus and carcinogenesis, contemporary problems, 564 588-9 historical aspects, 563--4 human foods (com, cotton seeds, peanuts, outbreaks, 57ll-1 tree nuts), 588 treatment and prevention. 577 Agaricus bisporus, 606 Bacillus cereus, 56&-75 AIDS see Acquired immunodeficiency antibodies, 574-5 syndrome characteristics, 564-5 Alcaligenes[aecalis, 343--4 chemical preservatives, 572 Allyl isothiocyanates, 694 detection, 573--4 Alternaria mycotoxins, 600 growth and survival, 572 Amanita spp. toxins (amanitins, growth temperature, 568 phallotoxins, virotoxins). 602-3 isolation, 573 ibotenic acid (lBA), 604 peR test, 574 isoxazoles, 605 prevalence in foods. 571-2 muscarine (MUS), 604-5 spore antibodies, 574-5 Amnesic shellfish poisoning (domoic acid), spores, germination, 572 676,682--4 spores, heat resistance of, 572 Pseudo-nitzschia spp., 682-3 virulence -

Plant-Based Medicines for Anxiety Disorders, Part 2: a Review of Clinical Studies with Supporting Preclinical Evidence
CNS Drugs 2013; 24 (5) Review Article Running Header: Plant-Based Anxiolytic Psychopharmacology Plant-Based Medicines for Anxiety Disorders, Part 2: A Review of Clinical Studies with Supporting Preclinical Evidence Jerome Sarris,1,2 Erica McIntyre3 and David A. Camfield2 1 Department of Psychiatry, Faculty of Medicine, University of Melbourne, Richmond, VIC, Australia 2 The Centre for Human Psychopharmacology, Swinburne University of Technology, Melbourne, VIC, Australia 3 School of Psychology, Charles Sturt University, Wagga Wagga, NSW, Australia Correspondence: Jerome Sarris, Department of Psychiatry and The Melbourne Clinic, University of Melbourne, 2 Salisbury Street, Richmond, VIC 3121, Australia. Email: [email protected], Acknowledgements Dr Jerome Sarris is funded by an Australian National Health & Medical Research Council fellowship (NHMRC funding ID 628875), in a strategic partnership with The University of Melbourne, The Centre for Human Psychopharmacology at the Swinburne University of Technology. Jerome Sarris, Erica McIntyre and David A. Camfield have no conflicts of interest that are directly relevant to the content of this article. 1 Abstract Research in the area of herbal psychopharmacology has revealed a variety of promising medicines that may provide benefit in the treatment of general anxiety and specific anxiety disorders. However, a comprehensive review of plant-based anxiolytics has been absent to date. Thus, our aim was to provide a comprehensive narrative review of plant-based medicines that have clinical and/or preclinical evidence of anxiolytic activity. We present the article in two parts. In part one, we reviewed herbal medicines for which only preclinical investigations for anxiolytic activity have been performed. In this current article (part two), we review herbal medicines for which there have been both preclinical and clinical investigations for anxiolytic activity. -

Nicotine Induces Polyspermy in Sea Urchin Eggs Through a Non-Cholinergic Pathway Modulating Actin Dynamics
cells Article Nicotine Induces Polyspermy in Sea Urchin Eggs through a Non-Cholinergic Pathway Modulating Actin Dynamics 1,2 1, 2 1, Nunzia Limatola , Filip Vasilev y, Luigia Santella and Jong Tai Chun * 1 Department of Biology and Evolution of Marine Organisms, Stazione Zoologica Anton Dohrn, I-80121 Napoli, Italy; [email protected] (N.L.); [email protected] (F.V.) 2 Department of Research Infrastructures for Marine Biological Resources, Stazione Zoologica Anton Dohrn, I-80121 Napoli, Italy; [email protected] * Correspondence: [email protected] Current address: Centre de Recherche du Centre Hospitalier de l’Université de Montreal (CRCHUM) y Montreal, QC H2X 0A9, Canada. Received: 8 November 2019; Accepted: 21 December 2019; Published: 25 December 2019 Abstract: While alkaloids often exert unique pharmacological effects on animal cells, exposure of sea urchin eggs to nicotine causes polyspermy at fertilization in a dose-dependent manner. Here, we studied molecular mechanisms underlying the phenomenon. Although nicotine is an agonist of ionotropic acetylcholine receptors, we found that nicotine-induced polyspermy was neither mimicked by acetylcholine and carbachol nor inhibited by specific antagonists of nicotinic acetylcholine receptors. Unlike acetylcholine and carbachol, nicotine uniquely induced drastic rearrangement of egg cortical microfilaments in a dose-dependent way. Such cytoskeletal changes appeared to render the eggs more receptive to sperm, as judged by the significant alleviation of polyspermy by latrunculin-A and mycalolide-B. In addition, our fluorimetric assay provided the first evidence that nicotine directly accelerates polymerization kinetics of G-actin and attenuates depolymerization of preassembled F-actin. Furthermore, nicotine inhibited cofilin-induced disassembly of F-actin.