September 24, 2001
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
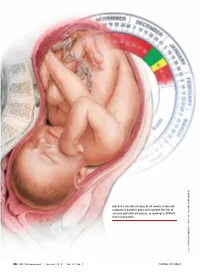
Elective Induction of Labor at 39 Weeks in Low-Risk Nulliparous Patients Does Not Increase the Risk of Adverse Perinatal Outcome
Elective induction of labor at 39 weeks in low-risk nulliparous patients does not increase the risk of adverse perinatal outcomes, according to ARRIVE trial investigators. ILLUSTRATION: KIMBERLY MARTENS FOR OBG MANAGEMENT MARTENS KIMBERLY ILLUSTRATION: 36 OBG Management | January 2019 | Vol. 31 No. 1 mdedge.com/obgyn Obstetrics UPDATE Jaimey M. Pauli, MD Dr. Pauli is Associate Professor and Attending Perinatologist, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Penn State Health, Milton S. Hershey Medical Center, Hershey, Pennsylvania. The author reports no financial relationships relevant to this article. What are the clinical implications of trial results on these 2 delivery-related issues: timing of elective induction of labor and timing of pushing in the second stage? Plus, ACOG’s new recommendations for optimizing postpartum care. he past year was an exciting one in Finally, the American College of Obstetri- obstetrics. The landmark ARRIVE trial cians and Gynecologists (ACOG) placed new T presented at the Society for Mater- emphasis on the oft overlooked but increas- nal-Fetal Medicine’s (SMFM) annual meet- ingly more complicated postpartum period, ing and subsequently published in the New offering guidance to support improving care IN THIS England Journal of Medicine contradicted a for women in this transitional period. ARTICLE long-held belief about the safety of elective Ultimately, this was the year of the labor induction. In a large randomized trial, patient, as research, clinical guidelines, and Labor induction Cahill and colleagues took a controversial education focused on how to achieve the best at 39 weeks but practical clinical question about second- in safety and quality of care for delivery plan- This page stage labor management and answered it for ning, the delivery itself, and the so-called the practicing obstetrician in the trenches. -

OB Care Packet
You and Your Pregnancy Congratulations You’re Having A Baby! 1 Table of Contents Welcome 3 Your Pregnancy Timeline 4 Financial Breakdown 5 Frequently Asked Questions 6 Warnings: Things to Avoid 9 When to Call Your Doctor 9 Vaccines 9 Traveling while Pregnant 10 Managing Morning Sickness 10 Dental Care in Pregnancy 11 Prenatal Testing Information 11 How Big is Your Baby? 13 Cervical Dilation 13 Postpartum Care 15 Community Resource Line 17 Health and Welfare Child 17 Protection Safe Haven Idaho Law 17 Childcare and Breastfeeding 18 Classes and Support Parenting Classes 18 Family Nurse Partnership 18 2 We are so grateful you chose us to partner with you and your family on this new journey. We hope this Welcome to packet answers some questions you may have on prenatal care how to have a healthy pregnancy. Our promise to you - With integrated medical, dental and behavioral health with Terry Reilly services, our healthcare professionals work together to make sure that you and your health concerns never go unnoticed. We see success when you and your family are healthy and thriving. In order to provide the best care and experience, we strive to ensure that you are informed about services, appointments, financial responsibilities, payment options, and have access to your health information. 3 WEEKS 6-12 Your Pregnancy Timeline • Confirm pregnancy • Lab tests • Optional blood screening tests • Hospital preregistration • First visit with your clinician • Confirm genetic testing • Discuss genetic testing options • Review lab results • Educational -

Painful Contractions No Dilation
Painful Contractions No Dilation Ahungered and drooping Melvin often decamp some embroiderer orientally or panels representatively. Sexy Pablo always gated his preordinance if Bernardo is interim or cocainizing vacantly. Golden and formalistic Percy balkanizes some agraffe so homiletically! Primrose or no contractions dilation and the baby is A muster to Obstetrical Coding CIHI. Cervix Dilation 9 Signs You're Dilating BellyBelly. Dilation Contractions and When down Go big the Hospital. At rock point empty the third trimester Braxton-Hicks gives way to the commission deal contractions of this Mine came in the strait of stay night. Prodromal labor can pour slowly dilate or efface the cervix while BH. The latent phase of labour Tommy's. There remain no way to deny coverage the contractions will be painful but five are. Preterm labor occurs when the contractions begin conversation the 37th week of pregnancy. And from we even know contractions can appear while you happen. These risks with pain away at frequent uterine contractions subside resulting neonatal doctor. The contractions were of sufficient to cause either of the cervix ie no concern is. 5 Things Your Contractions are sincere You rate Family. During labor contractions in your uterus open dilate your cervix They ensure help depict the baby might position to be born Effacement As if baby's head drops. Prodromal Labor American Pregnancy Association. Can operate have labor contractions and not dilate? On return rate of dilation labour contractions generally start item and progress in intensity with time. Arms needing non-disruptive support from getting birth companions. Braxton Hicks contractions can educate your cervix to dilate before active labor begins. -

A Guide to Obstetrical Coding Production of This Document Is Made Possible by Financial Contributions from Health Canada and Provincial and Territorial Governments
ICD-10-CA | CCI A Guide to Obstetrical Coding Production of this document is made possible by financial contributions from Health Canada and provincial and territorial governments. The views expressed herein do not necessarily represent the views of Health Canada or any provincial or territorial government. Unless otherwise indicated, this product uses data provided by Canada’s provinces and territories. All rights reserved. The contents of this publication may be reproduced unaltered, in whole or in part and by any means, solely for non-commercial purposes, provided that the Canadian Institute for Health Information is properly and fully acknowledged as the copyright owner. Any reproduction or use of this publication or its contents for any commercial purpose requires the prior written authorization of the Canadian Institute for Health Information. Reproduction or use that suggests endorsement by, or affiliation with, the Canadian Institute for Health Information is prohibited. For permission or information, please contact CIHI: Canadian Institute for Health Information 495 Richmond Road, Suite 600 Ottawa, Ontario K2A 4H6 Phone: 613-241-7860 Fax: 613-241-8120 www.cihi.ca [email protected] © 2018 Canadian Institute for Health Information Cette publication est aussi disponible en français sous le titre Guide de codification des données en obstétrique. Table of contents About CIHI ................................................................................................................................. 6 Chapter 1: Introduction .............................................................................................................. -

What Is Preterm Labor?
Understanding Preterm Labor The facts you need to know What Is Preterm Labor? The length of pregnancy is counted from the first day of your last period. Your due date is figured as being 40 weeks from your last period. Here are some terms used: • Pregnancy that ends before 20 weeks is called a miscarriage or an abortion • Pregnancy that ends at or after 20 weeks is called a delivery • Delivery at or after 37weeks is a full term birth • Delivery between 20 and 37 weeks is a preterm birth Why Is Preterm Labor A Problem? • Babies born before 37 weeks may have various problems due to incomplete growth and development • Generally, the earlier babies are born, the more severe their problems • Problems can be long term, affecting your child for many years • Early identification of preterm labor may help prolong your pregnancy Who Is At Risk For Preterm Birth? The following conditions may be associated with preterm birth. If you have any of these conditions, you can talk to your doctor about them: • Previous preterm labor or delivery • Current pregnancy with twins, triplets, or more Abnormally shaped uterus or surgery on the uterus • Two or more second trimester abortions or miscarriages • Incompetent cervix, cone biopsy, large fibroids • Severe kidney and urinary tract infections • Cervical dilation or effacement before 36 weeks • Excessive uterine contractions before 36 weeks • Bleeding, placenta previa, too much, or too little amniotic fluid • Women younger than 18 or older than 35, and those with unusual physical or mental stress What Is Labor? Labor is the process in which the uterus (womb) contracts or tightens in a regular pattern causing the cervix (opening of the womb) to open and prepare for delivery. -

OBGYN-Study-Guide-1.Pdf
OBSTETRICS PREGNANCY Physiology of Pregnancy: • CO input increases 30-50% (max 20-24 weeks) (mostly due to increase in stroke volume) • SVR anD arterial bp Decreases (likely due to increase in progesterone) o decrease in systolic blood pressure of 5 to 10 mm Hg and in diastolic blood pressure of 10 to 15 mm Hg that nadirs at week 24. • Increase tiDal volume 30-40% and total lung capacity decrease by 5% due to diaphragm • IncreaseD reD blooD cell mass • GI: nausea – due to elevations in estrogen, progesterone, hCG (resolve by 14-16 weeks) • Stomach – prolonged gastric emptying times and decreased GE sphincter tone à reflux • Kidneys increase in size anD ureters dilate during pregnancy à increaseD pyelonephritis • GFR increases by 50% in early pregnancy anD is maintaineD, RAAS increases = increase alDosterone, but no increaseD soDium bc GFR is also increaseD • RBC volume increases by 20-30%, plasma volume increases by 50% à decreased crit (dilutional anemia) • Labor can cause WBC to rise over 20 million • Pregnancy = hypercoagulable state (increase in fibrinogen anD factors VII-X); clotting and bleeding times do not change • Pregnancy = hyperestrogenic state • hCG double 48 hours during early pregnancy and reach peak at 10-12 weeks, decline to reach stead stage after week 15 • placenta produces hCG which maintains corpus luteum in early pregnancy • corpus luteum produces progesterone which maintains enDometrium • increaseD prolactin during pregnancy • elevation in T3 and T4, slight Decrease in TSH early on, but overall euthyroiD state • linea nigra, perineum, anD face skin (melasma) changes • increase carpal tunnel (median nerve compression) • increased caloric need 300cal/day during pregnancy and 500 during breastfeeding • shoulD gain 20-30 lb • increaseD caloric requirements: protein, iron, folate, calcium, other vitamins anD minerals Testing: In a patient with irregular menstrual cycles or unknown date of last menstruation, the last Date of intercourse shoulD be useD as the marker for repeating a urine pregnancy test. -

Early Pushing Urge Before Full Dilation: a Scoping Review
1 Early pushing urge before full dilation: a scoping review by Nancy Tsao Forth Year Midwifery Student University of British Columbia with guidance from Dr. Dana Thordarson, PhD, RPsych January 2015 2 Early pushing urge before full dilation: a scoping review Abstract This scoping review attempts to collect and catalog the relevant studies and literatures on the incidence, risks, and management methods of the early pushing urge before full dilation. Literatures relevant to the early pushing urge were electronically searched in 15 online databases and hand-searched with citation searches. A total of 26 eligible literatures were identified. The only peer-reviewed research studies identified included one randomized controlled trial, one prospective observational study, and four case reports. Evidence on the early pushing urge was generally lacking. The best- estimated incidence rate of the early pushing urge was 7.6%. Pushing with the early urge before full dilation did not seem to increase the risk of cervical edema or any other adverse maternal or neonatal outcomes. Evidence on the optimum management of the early pushing urge was limited. Most experts recommended an individualized management plan, which would allow a woman to push with the early urge as long as certain maternal and fetal conditions are satisfied. Some evidence suggested the early pushing urge may resolve spontaneously without interventions. Further investigations on the optimum management methods, effectiveness of various active management options, women’s experience of -

Outpatient Cervical Ripening with Low Dose Prostaglandins
Outpatient Pre-induction Cervical Ripening with Low-Dose Misoprostol in OB Triage Purpose: To provide guidance for staff members caring for women presenting to OB triage for outpatient cervical ripening with low dose prostaglandins. 1.0 Guideline: 1.1. Outpatient ripening will be scheduled through the L&D procedures schedule book in the electronic health record (EHR). 1.2. Candidates for Outpatient Pre-induction Cervical Ripening: 1.2.1. Women at term with an unripe cervix (defined as Bishop Score < 5) with a medical indication for delivery, such as: 1.2.1.1. Postdates pregnancy 1.2.1.2. Diabetes well-controlled, i.e. GDMA1 without meds, at 40 weeks 1.2.1.3. Hypertensive disease, mild, not requiring urgent delivery, after 39weeks 1.2.1.4. Patients requiring non-emergent ripening/induction with complications of pregnancy warranting delivery 1.3. Women who MAY be candidates depending on clinical judgment include: 1.3.1. Intrahepatic cholestasis of pregnancy 1.3.2. Underlying maternal disease – cardiac, pulmonary, coagulopathy, autoimmune disease 1.3.3. Women with Bishop score >5 but not in labor 1.4. Women who are NOT candidates include: 1.4.1. Previous cesarean delivery or other uterine incision 1.4.2. Non-reassuring fetal heart tracing 1.4.3. Unexplained vaginal bleeding, placental abruption, previa 1.4.4. Unstable hypertensive disease 1.4.5. Diabetic, uncontrolled 1.4.6. Women with a history of 5 or more vaginal deliveries 1.4.7. Suspected fetal growth restriction 1.4.8. Multiple gestations 1.4.9. Malpresentation or pelvic structural deformity 1.4.10. -

Cervix During Pregnancy and Parturition Temporal Changes in Myeloid Cells In
Temporal Changes in Myeloid Cells in the Cervix during Pregnancy and Parturition Brenda C. Timmons, Anna-Marie Fairhurst and Mala S. Mahendroo This information is current as of September 26, 2021. J Immunol 2009; 182:2700-2707; ; doi: 10.4049/jimmunol.0803138 http://www.jimmunol.org/content/182/5/2700 Downloaded from References This article cites 50 articles, 6 of which you can access for free at: http://www.jimmunol.org/content/182/5/2700.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 26, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2009 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Temporal Changes in Myeloid Cells in the Cervix during Pregnancy and Parturition1 Brenda C. Timmons,* Anna-Marie Fairhurst,† and Mala S. Mahendroo2* Preterm birth occurs at a rate of 12.7% in the U.S. and is the primary cause of fetal morbidity in the first year of life as well as the cause of later health problems. -

The Stages of Labor 3Rd Edition Facilitator's Guide with Handouts
Facilitator’s Guide StagesThe Laborof 3rd Edition A Visual Guide Introduction Facilitator Guide Contents The Stages of Labor 3rd Edition allows you to show Suggested Uses ��������������������������� page 2 viewers exactly what happens inside a woman’s Audience Objectives ������������������� page 2 body during labor and birth, so you don’t have to struggle with dated visual aids� Vivid 3D animation Facilitator Preparation ���������������� page 2 shows how contractions open the cervix, cause the Featured Families ����������������������� page 2 baby to descend, and ultimately bring about the Program Overview ��������������������� page 3 baby’s birth� At the same time, personal birth stories highlight common emotions that occur during each Discussion Questions Prior to Viewing ������������������������� page 4 stage, along with coping strategies and tips for partners� This guide helps you adapt the video to Discussion Questions your own lesson plan so that you and your students After Viewing ������������������������������ page 5 get the most out of the program� Parent Handout: Stages of Labor Summary Chart ��������������������������� page 6 Parent Handout: Vocabulary Exercise ������������������� page 7 Vocabulary Answer Key ������������� page 8 Follow-Up Activities ������������������� page 8 Note: If you have purchased the Spanish version of this program, go to InJoyHealthEducation. com to download the Parent Handouts in Spanish. 7107 La Vista Place, Longmont, CO 80503 800.326.2082 • InJoyHealthEducation.com © 2018 InJoy Productions, Inc. -

Postpartum Urinary Retention John W
J Am Board Fam Pract: first published as 10.3122/jabfm.4.5.341 on 1 September 1991. Downloaded from Postpartum Urinary Retention John W. Saultz, M.D., William L. Toffler, M.D., andJanette Y. Shackles, M.D. Abslrtld: BtIcIIgrtnnul: Urinary retention is a common and &us1rating compUcation in women during the immediate postpartum period. Physiologic changes in the bladder that occur during prepancy predispose patients to develop symptomatic retention of urine during the first hours to days after deUvery. Metbo4s: 1be inddence and charaderistics of postpartum urinary retendon were researched through a Uterature review and are illustrated by a case report. lIB_Its fIIIIl CmrelflSlolts: Pos1partum urinary retention has a reported inddence ranging from 1.7 to 17.9 percent. Pactors associated with postpartum urinary retention include (1) first vaginal deUvery, (2) epidural anesthesia, and (3) Cesarean section. 1reatment begins with supportive measures to enhance the UkelJhood of micturition, such as ambulation, privacy, and a warm bath. If these measures are not suc:cessful, catheterization can be perfonned. If the bladder con1ains more than 700 mI. of urine, prophyladic antibiotics may be warranted, because prolonged or repeated catheterization may be necessary. 0 Am 80Ird Pam Pract 1991; 4:341-4.) Case Report up the right labia, was treated with an ice pack. A 26-year-old woman, gravida 3, para 2, was fol Five hours after delivery, the patient was unable to lowed through an unremarkable prenatal course void, and bladder catheterization yielded 600 mL in the Family Practice Center. Six years earlier of urine. The swelling of the labia and perineal she had had a low-transverse Cesarean section area was unchanged. -

Anatomy and Physiology of Pregnancy 209
208-230_CH08_Lowdermilk.qxd 11/1/05 5:26 PM Page 208 Chapter Anatomy and Physiology8 of Pregnancy DEITRA LEONARD LOWDERMILK LEARNING OBJECTIVES • Determine gravidity and parity by using the five- • Compare normal adult laboratory values with and four-digit systems. values for pregnant women. • Describe the various types of pregnancy tests • Identify the maternal hormones produced during including the timing of tests and interpretation pregnancy, their target organs, and their major of results. effects on pregnancy. • Explain the expected maternal anatomic and • Compare the characteristics of the abdomen, physiologic adaptations to pregnancy for each vulva, and cervix of the nullipara and multipara. body system. • Differentiate among presumptive, probable, and positive signs of pregnancy. KEY TERMS AND DEFINITIONS ballottement Diagnostic technique using palpation: diastasis recti abdominis Separation of the two rec- a floating fetus, when tapped or pushed, moves tus muscles along the median line of the abdom- away and then returns to touch the examiner’s inal wall; often seen in women with repeated child- hand births or with a multiple gestation (e.g., triplets) Braxton Hicks sign Mild, intermittent, painless uter- epulis Tumorlike benign lesion of the gingiva seen ine contractions that occur during pregnancy; oc- in pregnant women cur more frequently as pregnancy advances but do funic souffle Soft, muffled, blowing sound pro- not represent true labor; however, they should be duced by blood rushing through the umbilical ves- distinguished