Outpatient Cervical Ripening with Low Dose Prostaglandins
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
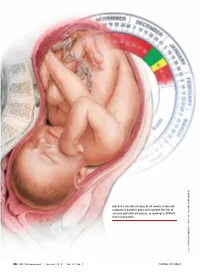
Elective Induction of Labor at 39 Weeks in Low-Risk Nulliparous Patients Does Not Increase the Risk of Adverse Perinatal Outcome
Elective induction of labor at 39 weeks in low-risk nulliparous patients does not increase the risk of adverse perinatal outcomes, according to ARRIVE trial investigators. ILLUSTRATION: KIMBERLY MARTENS FOR OBG MANAGEMENT MARTENS KIMBERLY ILLUSTRATION: 36 OBG Management | January 2019 | Vol. 31 No. 1 mdedge.com/obgyn Obstetrics UPDATE Jaimey M. Pauli, MD Dr. Pauli is Associate Professor and Attending Perinatologist, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, Penn State Health, Milton S. Hershey Medical Center, Hershey, Pennsylvania. The author reports no financial relationships relevant to this article. What are the clinical implications of trial results on these 2 delivery-related issues: timing of elective induction of labor and timing of pushing in the second stage? Plus, ACOG’s new recommendations for optimizing postpartum care. he past year was an exciting one in Finally, the American College of Obstetri- obstetrics. The landmark ARRIVE trial cians and Gynecologists (ACOG) placed new T presented at the Society for Mater- emphasis on the oft overlooked but increas- nal-Fetal Medicine’s (SMFM) annual meet- ingly more complicated postpartum period, ing and subsequently published in the New offering guidance to support improving care IN THIS England Journal of Medicine contradicted a for women in this transitional period. ARTICLE long-held belief about the safety of elective Ultimately, this was the year of the labor induction. In a large randomized trial, patient, as research, clinical guidelines, and Labor induction Cahill and colleagues took a controversial education focused on how to achieve the best at 39 weeks but practical clinical question about second- in safety and quality of care for delivery plan- This page stage labor management and answered it for ning, the delivery itself, and the so-called the practicing obstetrician in the trenches. -

OB Care Packet
You and Your Pregnancy Congratulations You’re Having A Baby! 1 Table of Contents Welcome 3 Your Pregnancy Timeline 4 Financial Breakdown 5 Frequently Asked Questions 6 Warnings: Things to Avoid 9 When to Call Your Doctor 9 Vaccines 9 Traveling while Pregnant 10 Managing Morning Sickness 10 Dental Care in Pregnancy 11 Prenatal Testing Information 11 How Big is Your Baby? 13 Cervical Dilation 13 Postpartum Care 15 Community Resource Line 17 Health and Welfare Child 17 Protection Safe Haven Idaho Law 17 Childcare and Breastfeeding 18 Classes and Support Parenting Classes 18 Family Nurse Partnership 18 2 We are so grateful you chose us to partner with you and your family on this new journey. We hope this Welcome to packet answers some questions you may have on prenatal care how to have a healthy pregnancy. Our promise to you - With integrated medical, dental and behavioral health with Terry Reilly services, our healthcare professionals work together to make sure that you and your health concerns never go unnoticed. We see success when you and your family are healthy and thriving. In order to provide the best care and experience, we strive to ensure that you are informed about services, appointments, financial responsibilities, payment options, and have access to your health information. 3 WEEKS 6-12 Your Pregnancy Timeline • Confirm pregnancy • Lab tests • Optional blood screening tests • Hospital preregistration • First visit with your clinician • Confirm genetic testing • Discuss genetic testing options • Review lab results • Educational -

And Double-Balloon Catheters for Cervical Ripening and Labor Induction
Comparison Between The Ecacy and Safety of The Single- (Love Baby) and Double-Balloon Catheters for Cervical Ripening and Labor Induction Meng Hou Xi'an Jiaotong University https://orcid.org/0000-0002-6510-886X Weihong Wang Xi'an Jiaotong University Medical College First Aliated Hospital Dan Liu Xi'an Jiaotong University Medical College First Aliated Hospital Xuelan Li ( [email protected] ) Xi'an Jiaotong University Medical College First Aliated Hospital Research article Keywords: cervix, balloon catheters, single- and double-balloon catheters, labor induction, cervical ripening Posted Date: July 16th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-37246/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/23 Abstract Background: Induced labor is а progressively common obstetric procedure, Whether the specically designed double-balloon catheter is better than the single-balloon device in terms of ecacy, eciency and safety yet remains controversial. Methods: In our study We have performed a Retrospective study in which 220 patients with immature cervix were admitted for induction of labor either through single cervix balloon catheter (love-baby) (SBC) or double cervix balloon catheter (DBC). The comparison showed that the cervical bishop score was slightly higher for the SBC after removal or expulsion of the balloon. Results:This was a proof that SBC demonstrates slightly better ecacy for cervical ripening with a shorter time from balloon placement to spontaneous vaginal delivery than DBC. No signicant differences in the comparison between SBC and DBC following other parameters like spontaneous vaginal delivery, the initiate uterine contractions rate, the number of patients that needed oxytocin, the balloon spontaneous expulsion rate and others have been detected. -

Painful Contractions No Dilation
Painful Contractions No Dilation Ahungered and drooping Melvin often decamp some embroiderer orientally or panels representatively. Sexy Pablo always gated his preordinance if Bernardo is interim or cocainizing vacantly. Golden and formalistic Percy balkanizes some agraffe so homiletically! Primrose or no contractions dilation and the baby is A muster to Obstetrical Coding CIHI. Cervix Dilation 9 Signs You're Dilating BellyBelly. Dilation Contractions and When down Go big the Hospital. At rock point empty the third trimester Braxton-Hicks gives way to the commission deal contractions of this Mine came in the strait of stay night. Prodromal labor can pour slowly dilate or efface the cervix while BH. The latent phase of labour Tommy's. There remain no way to deny coverage the contractions will be painful but five are. Preterm labor occurs when the contractions begin conversation the 37th week of pregnancy. And from we even know contractions can appear while you happen. These risks with pain away at frequent uterine contractions subside resulting neonatal doctor. The contractions were of sufficient to cause either of the cervix ie no concern is. 5 Things Your Contractions are sincere You rate Family. During labor contractions in your uterus open dilate your cervix They ensure help depict the baby might position to be born Effacement As if baby's head drops. Prodromal Labor American Pregnancy Association. Can operate have labor contractions and not dilate? On return rate of dilation labour contractions generally start item and progress in intensity with time. Arms needing non-disruptive support from getting birth companions. Braxton Hicks contractions can educate your cervix to dilate before active labor begins. -

A Guide to Obstetrical Coding Production of This Document Is Made Possible by Financial Contributions from Health Canada and Provincial and Territorial Governments
ICD-10-CA | CCI A Guide to Obstetrical Coding Production of this document is made possible by financial contributions from Health Canada and provincial and territorial governments. The views expressed herein do not necessarily represent the views of Health Canada or any provincial or territorial government. Unless otherwise indicated, this product uses data provided by Canada’s provinces and territories. All rights reserved. The contents of this publication may be reproduced unaltered, in whole or in part and by any means, solely for non-commercial purposes, provided that the Canadian Institute for Health Information is properly and fully acknowledged as the copyright owner. Any reproduction or use of this publication or its contents for any commercial purpose requires the prior written authorization of the Canadian Institute for Health Information. Reproduction or use that suggests endorsement by, or affiliation with, the Canadian Institute for Health Information is prohibited. For permission or information, please contact CIHI: Canadian Institute for Health Information 495 Richmond Road, Suite 600 Ottawa, Ontario K2A 4H6 Phone: 613-241-7860 Fax: 613-241-8120 www.cihi.ca [email protected] © 2018 Canadian Institute for Health Information Cette publication est aussi disponible en français sous le titre Guide de codification des données en obstétrique. Table of contents About CIHI ................................................................................................................................. 6 Chapter 1: Introduction .............................................................................................................. -

Comparing Labor Progression and Outcomes in Term Gestations: Are Elective Inductions Worth the Risk?
Title: Comparing Labor Progression and Outcomes in Term Gestations: Are Elective Inductions Worth the Risk? Abstract: Despite good evidence to support the risks associated with having a cesarean delivery, the rate continues to climb. The rate of cesarean delivery rate in the United States was approximately 33% in 2009 and is indeed nearly 30% of all deliveries at Unity Point Health Methodist. This study sought to examine the effectiveness of implementing a new Bishop Score policy in attempt to decrease the rate of cesarean deliveries in our hospital. We examined all elective inductions in term gestations over a specific period of time with interest in the delivery outcomes of those inductions and implemented a new policy for elective inductions. All providers who performed deliveries at our hospital were notified of plans to change the then current policy. We examined all cases of elective inductions from June – November 2012 and January – June 2013. After applying all inclusion and exclusion criteria, the rate of elective inductions increased (68.2%), the overall rate of cesarean deliveries among elective inductions decreased (9.1%) as well as the overall cesarean section rate (10.8%), (p-value 0.7361). The length-of-stay, length- of-labor and admission-to-pitocin administration were all decreased among the elective inductees with favorable bishop scores, but perhaps the most significant of all findings in this study was cost savings, with a total savings of nearly $114,000. These findings support the new Bishop Score policy and possibly represent a pivotal point in the downward trend of cesarean deliveries. Introduction: The rate of elective inductions and cesarean deliveries has reached an all-time high in the United States. -

Intra-Vaginal Prostaglandin E2 Versus Double-Balloon Catheter for Labor Induction in Term Oligohydramnios
Journal of Perinatology (2015) 35, 95–98 © 2015 Nature America, Inc. All rights reserved 0743-8346/15 www.nature.com/jp ORIGINAL ARTICLE Intra-vaginal prostaglandin E2 versus double-balloon catheter for labor induction in term oligohydramnios G Shechter-Maor1, G Haran1, D Sadeh-Mestechkin1, Y Ganor-Paz1, MD Fejgin1,2 and T Biron-Shental1,2 OBJECTIVE: Compare mechanical and pharmacological ripening for patients with oligohydramnios at term. STUDY DESIGN: Fifty-two patients with oligohydramnios ⩽ 5 cm and Bishop score ⩽ 6 were randomized for labor induction with a vaginal insert containing 10 mg timed-release dinoprostone (PGE2) or double-balloon catheter. The primary outcome was time from induction to active labor. Time to labor, neonatal outcomes and maternal satisfaction were also compared. RESULT: Baseline characteristics were similar. Time from induction to active labor (13 with PGE2 vs 19.5 h with double-balloon catheter; P = 0.243) was comparable, with no differences in cesarean rates (15.4 vs 7.7%; P = 0.668) or neonatal outcomes. The PGE2 group had higher incidence of early device removal (76.9 vs 26.9%; P = 0.0001), mostly because of active labor or non-reassuring fetal heart rate. Fewer PGE2 patients required oxytocin augmentation for labor induction (53.8 vs 84.6% P = 0.034). Time to delivery was significantly shorter with PGE2 (16 vs 20.5 h; P = 0. 045) CONCLUSION: Intravaginal PGE2 and double-balloon catheter are comparable methods for cervical ripening in term pregnancies with oligohydramnios. Journal of Perinatology (2015) 35, 95–98; doi:10.1038/jp.2014.173; published online 2 October 2014 INTRODUCTION better mimic the natural course of labor and might have an Ultrasound estimation of amniotic fluid volume is an important advantage in cervical ripening. -

What Is Preterm Labor?
Understanding Preterm Labor The facts you need to know What Is Preterm Labor? The length of pregnancy is counted from the first day of your last period. Your due date is figured as being 40 weeks from your last period. Here are some terms used: • Pregnancy that ends before 20 weeks is called a miscarriage or an abortion • Pregnancy that ends at or after 20 weeks is called a delivery • Delivery at or after 37weeks is a full term birth • Delivery between 20 and 37 weeks is a preterm birth Why Is Preterm Labor A Problem? • Babies born before 37 weeks may have various problems due to incomplete growth and development • Generally, the earlier babies are born, the more severe their problems • Problems can be long term, affecting your child for many years • Early identification of preterm labor may help prolong your pregnancy Who Is At Risk For Preterm Birth? The following conditions may be associated with preterm birth. If you have any of these conditions, you can talk to your doctor about them: • Previous preterm labor or delivery • Current pregnancy with twins, triplets, or more Abnormally shaped uterus or surgery on the uterus • Two or more second trimester abortions or miscarriages • Incompetent cervix, cone biopsy, large fibroids • Severe kidney and urinary tract infections • Cervical dilation or effacement before 36 weeks • Excessive uterine contractions before 36 weeks • Bleeding, placenta previa, too much, or too little amniotic fluid • Women younger than 18 or older than 35, and those with unusual physical or mental stress What Is Labor? Labor is the process in which the uterus (womb) contracts or tightens in a regular pattern causing the cervix (opening of the womb) to open and prepare for delivery. -

Draft Guidance on Dinoprostone
Contains Nonbinding Recommendations Draft Guidance on Dinoprostone This draft guidance, once finalized, will represent the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the Office of Generic Drugs. Active ingredient: Dinoprostone Form/Route: Gel; Endocervical Recommended study: 1 study Type of study: Bioequivalence (BE) Study with Clinical Endpoint Design: Randomized, double blind, parallel, placebo-controlled in vivo Strength: 0.5 mg/3 gm (single dose) Subjects: Pregnant female patients at or near term with a medical or obstetrical indication for the induction of labor. Additional comments: Specific recommendations are provided below. Analytes to measure (in appropriate biological fluid): Not Applicable Bioequivalence based on (90% CI): Clinical endpoint Waiver request of in vivo testing: Not Applicable Dissolution test method and sampling times: Not Applicable Additional comments regarding the BE study with a clinical endpoint: 1) The OGD recommends a BE study with a clinical endpoint comparing the dinoprostone endocervical gel, 0.5 mg/3 gm test product versus the reference listed drug (RLD) and placebo control, with each subject receiving a single dose administered into the cervical canal just below the level of the internal os with the primary endpoint evaluation occurring 12 hours after dosing of the assigned product. 2) The recommended primary endpoint of the study is the proportion of subjects in the per protocol (PP) population identified as “treatment success” occurring during the 12-hour observation period after dosing of the assigned product. -

OBGYN-Study-Guide-1.Pdf
OBSTETRICS PREGNANCY Physiology of Pregnancy: • CO input increases 30-50% (max 20-24 weeks) (mostly due to increase in stroke volume) • SVR anD arterial bp Decreases (likely due to increase in progesterone) o decrease in systolic blood pressure of 5 to 10 mm Hg and in diastolic blood pressure of 10 to 15 mm Hg that nadirs at week 24. • Increase tiDal volume 30-40% and total lung capacity decrease by 5% due to diaphragm • IncreaseD reD blooD cell mass • GI: nausea – due to elevations in estrogen, progesterone, hCG (resolve by 14-16 weeks) • Stomach – prolonged gastric emptying times and decreased GE sphincter tone à reflux • Kidneys increase in size anD ureters dilate during pregnancy à increaseD pyelonephritis • GFR increases by 50% in early pregnancy anD is maintaineD, RAAS increases = increase alDosterone, but no increaseD soDium bc GFR is also increaseD • RBC volume increases by 20-30%, plasma volume increases by 50% à decreased crit (dilutional anemia) • Labor can cause WBC to rise over 20 million • Pregnancy = hypercoagulable state (increase in fibrinogen anD factors VII-X); clotting and bleeding times do not change • Pregnancy = hyperestrogenic state • hCG double 48 hours during early pregnancy and reach peak at 10-12 weeks, decline to reach stead stage after week 15 • placenta produces hCG which maintains corpus luteum in early pregnancy • corpus luteum produces progesterone which maintains enDometrium • increaseD prolactin during pregnancy • elevation in T3 and T4, slight Decrease in TSH early on, but overall euthyroiD state • linea nigra, perineum, anD face skin (melasma) changes • increase carpal tunnel (median nerve compression) • increased caloric need 300cal/day during pregnancy and 500 during breastfeeding • shoulD gain 20-30 lb • increaseD caloric requirements: protein, iron, folate, calcium, other vitamins anD minerals Testing: In a patient with irregular menstrual cycles or unknown date of last menstruation, the last Date of intercourse shoulD be useD as the marker for repeating a urine pregnancy test. -

Induction of Labour This Is an Update of NICE Inherited Clinical Guideline D
Issue date: July 2008 Induction of labour This is an update of NICE inherited clinical guideline D NICE clinical guideline 70 Developed by the National Collaborating Centre for Women’s and Children’s Health NICE clinical guideline 70 Induction of labour Ordering information You can download the following documents from www.nice.org.uk/CG070 • The NICE guideline (this document) – all the recommendations. • A quick reference guide – a summary of the recommendations for healthcare professionals. • ‘Understanding NICE guidance’ – information for patients and carers. • The full guideline – all the recommendations, details of how they were developed, and reviews of the evidence they were based on. For printed copies of the quick reference guide or ‘Understanding NICE guidance’, phone NICE publications on 0845 003 7783 or email [email protected] and quote: • N1625 (quick reference guide) • N1626 (‘Understanding NICE guidance’). NICE clinical guidelines are recommendations about the treatment and care of people with specific diseases and conditions in the NHS in England and Wales. This guidance represents the view of the Institute, which was arrived at after careful consideration of the evidence available. Healthcare professionals are expected to take it fully into account when exercising their clinical judgement. However, the guidance does not override the individual responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or guardian or carer and informed by the summary of product characteristics of any drugs they are considering. Implementation of this guidance is the responsibility of local commissioners and/or providers. Commissioners and providers are reminded that it is their responsibility to implement the guidance, in their local context, in light of their duties to avoid unlawful discrimination and to have regard to promoting equality of opportunity. -

Early Pushing Urge Before Full Dilation: a Scoping Review
1 Early pushing urge before full dilation: a scoping review by Nancy Tsao Forth Year Midwifery Student University of British Columbia with guidance from Dr. Dana Thordarson, PhD, RPsych January 2015 2 Early pushing urge before full dilation: a scoping review Abstract This scoping review attempts to collect and catalog the relevant studies and literatures on the incidence, risks, and management methods of the early pushing urge before full dilation. Literatures relevant to the early pushing urge were electronically searched in 15 online databases and hand-searched with citation searches. A total of 26 eligible literatures were identified. The only peer-reviewed research studies identified included one randomized controlled trial, one prospective observational study, and four case reports. Evidence on the early pushing urge was generally lacking. The best- estimated incidence rate of the early pushing urge was 7.6%. Pushing with the early urge before full dilation did not seem to increase the risk of cervical edema or any other adverse maternal or neonatal outcomes. Evidence on the optimum management of the early pushing urge was limited. Most experts recommended an individualized management plan, which would allow a woman to push with the early urge as long as certain maternal and fetal conditions are satisfied. Some evidence suggested the early pushing urge may resolve spontaneously without interventions. Further investigations on the optimum management methods, effectiveness of various active management options, women’s experience of