Journal of Environmental Science, Computer Science and Engineering & Technology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Project Staff
Project Staff Thanhlupuia : Research Officer Ruth Lalrinsangi : Inspector of Statistics Lalrinawma : Inspector of Statistics Zorammawii Colney : Software i/c Lalrintluanga : Software i/c Vanlalruati : Statistical Cell Contents Page No. 1. Foreword - (i) 2. Preface - (ii) 3. Message - (iii) 4. Notification - (iv) Part-A (Abstract) 1. Dept. of School Education, Mizoram 2009-2010 at a Glance - 1 2. Number of schools by management - 2 3. Enrolment of students by management-wise - 3 4. Number of teachers by management-wise - 4 5. Abstract of Primary Schools under Educational Sub-Divisions - 5-9 6. Abstract of Middle Schools under Educational Sub-Divisions - 10-16 7. Abstract of High Schools under Educational Districts - 17-18 8. Abstract of Higher Secondary Schools under Educational Districts - 19-23 Part-B (List of Schools with number of teachers and enrolment of students) PRIMARY SCHOOLS: Aizawl District 1.SDEO, AizawlEast - 25-30 2.SDEO, AizawlSouth - 31-33 3.SDEO, AizawlWest - 34-38 4. SDEO, Darlawn - 39-41 5.SDEO, Saitual - 42-43 Champhai District 6.SDEO, Champhai - 44-47 7. SDEO, Khawzawl - 48-50 Kolasib District 8. SDEO, Kolasib - 51-53 9. SDEO, Kawnpui - 54-55 Lawngtlai District 10. EO, CADC - 56-59 11. EO, LADC - 60-64 Lunglei District 12.SDEO, LungleiNorth - 65-67 13.SDEO, LungleiSouth - 68-70 14.SDEO, Lungsen - 71-74 15. SDEO, Hnahthial - 75-76 Mamit District 16. SDEO, Mamit - 77-78 17. SDEO, Kawrthah - 79-80 18.SDEO, WestPhaileng - 81-83 Saiha District 19. EO, MADC - 84-87 Serchhip District 20. SDEO, Serchhip - 88-89 21. SDEO, North Vanlaiphai - 90 22.SDEO, Thenzawl - 91 MIDDLE SCHOOLS: Aizawl District 23.SDEO, Aizawl East - 93-97 24.SDEO, AizawlSouth - 98-99 25. -

The Mizoram Gazette Published by Authority
The Mizoram Gazette EXTRA ·ORDINARY Published by Authority REGN. NO. N.E.-313 (MZ) Rs. 2/- per Issue VOL. XXX" Aizawl. Wednesday, 26.4.2006, Valsakha 6, S.E. 1928, Issue No. 103 NOTfCATION No. B. 14016/3/02-LAD/VC, the 18th April, 2006. In exercise of the powers co nferred by Section 7(1 )(2), Section 15 and Section 22(1) of the Lusbai Hills District (Village Councils) Act, 1953, as amended from time to time, the Gover- nor of Mizoram is pleased to approve Executive Body of the following Village Councils as shown in the enclosed Annexure within Cha mphai District. R. Sangliankhuma, Addl. Secretary, Local Administration Department. ANNEXURE CHAMPHAI DISTRICT -........------;-------- ----- 1 2 3 4 I4-KHAWBUNG Ale 1. Buang 1. Tinlinga· President 2. Thangchhinga Vice President & Treasurer 3. K. Lalzawngliana Secretary 4. ParlaV\oma Crier Ex-103/20C6 2 1 2 3 4 2. Bungzung J. Lalringzuala President 2. Lalpianfa Vice Pr�sider.t 3. La]zapa Trt:asurer 4. Lalhmingliana Secretary 5. Sangkhuma Crier 3. Bulfekzawl 1. Vanlalrin ga President 2. Chhunkhuma Vice Presifient 3. Chh un tb angvunga Treasurer 4. Laltums2nga Seer etary 5. I. F. }.'Iallga Crier 4. Chawngtui -E' Not yet formed Executive Body 5. Dungtlang 1. H. Laldin gliana President ., .. C. Zalawma Vice President 3. K. LaIthlamuana Treasurer 4. C. Lalzama Secretary 5. Kaprothanga Crier l 6. Farkawn 1. Lalslama President 2. Manghupa Vice President 3. Engm av. ia Treasurer 4. Ljansailova Secretary 5. Lalrawna Crier 7. Hruaikawn 1. C. Hranghleikapa President 2. Sangtinkulha Vice President 3. Sa ngtha n gpuia Treasurer 4 . -

Gender Role in the Livelihood Activities of Shifting Cultivation in Mizoram: a Case Study of Chawngtlai Village Gender Role in T
GENDER ROLE IN THE LIVELIHOOD ACTIVITIES OF SHIFTING CULTIVATION IN MIZORAM: A CASE STUDY OF CHAWNGTLAI VILLAGE A Dissertation submitted in partial fulfillment for the award of the degree of Master of philosophy in Economics By AGNES LALREMRUATI To DEPARTMENT OF ECONOMICS MIZORAM UNIVERSITY TANHRIL-796004 2016 GENDER ROLE IN THE LIVELIHOOD ACTIVITIES OF SHIFTING CULTIVATION IN MIZORAM: A CASE STUDY OF CHAWNGTLAI VILLAGE 1 A Dissertation submitted in partial fulfillment for the award of the degree of Master of philosophy in Economics By AGNES LALREMRUATI MZU/M.Phil./281 of 22.04.2016 Supervisor Dr. James L.T Thanga DEPARTMENT OF ECONOMICS MIZORAM UNIVERSITY TANHRIL-796004 2016 II 2 MIZORAM UNIVERSITY DEPARTMENT OF ECONOMICS AIZAWL, MIZORAM-796004, Phone: 0389-2330708/2330709, Fax: 0389-2330709 CERTIFICATE This is to certify that Agnes Lalremruati has carried out the research embodied in the present dissertation entitled “Gender Role in the Livelihood Activities of Shifting Cultivation in Mizoram: A Case Study of Chawngtlai Village” submitted in partial fulfillment of the requirement for the award of the Degree of Master of Philosophy in Economics, a full time student in the Department of Economics, Mizoram University during the Academic year 2015-2016. This dissertation has not previously formed the basis for the award of any Degree. This dissertation is an independent work of the candidate but for the guidance provided by me. (Dr. JAMES L.T THANGA) Supervisor Date: ……….. III MIZORAM UNIVERSITY DECEMBER, 2016 3 DECLARATION I, Agnes Lalremruati, hereby declare that the dissertation “Gender Role in the Livelihood Activities of Shifting Cultivation in Mizoram: A Case Study of Chawngtlai Village” being the title of the dissertation submitted by me for the Degree of Master of Philosophy in Economics is a record of research work done by me during the academic year 2015-2016 and that the dissertation has not formed the basis for the award of any Degree, Diploma, Fellowship, or other similar title. -
SELESIH 2 AIZAWL EAST Aizawl
Sl No DCSO Village_Name_EN FPS_ShopName_EN 1 AIZAWL EAST Aizawl (NT) SELESIH 2 AIZAWL EAST Aizawl (NT) DURTLANG NORTH 3 AIZAWL EAST Aizawl (NT) DURTLANG - I 4 AIZAWL EAST Aizawl (NT) DURTLANG - II 5 AIZAWL EAST Aizawl (NT) DURTLANG LEITAN - I 6 AIZAWL EAST Aizawl (NT) DURTLANG LEITAN - II 7 AIZAWL EAST Aizawl (NT) BAWNGKAWN - I 8 AIZAWL EAST Aizawl (NT) BAWNGKAWN - II 9 AIZAWL EAST Aizawl (NT) BAWNGKAWN - III 10 AIZAWL EAST Aizawl (NT) BAWNGKAWN SOUTH 11 AIZAWL EAST Aizawl (NT) CHALTLANG - I 12 AIZAWL EAST Aizawl (NT) CHALTLANG - II 13 AIZAWL EAST Aizawl (NT) CHALTLANG - III 14 AIZAWL EAST Aizawl (NT) CHALTLANG -IV 15 AIZAWL EAST Aizawl (NT) LAIPUITLANG 16 AIZAWL EAST Aizawl (NT) RAMHLUN NORTH - I 17 AIZAWL EAST Aizawl (NT) RAMHLUN NORTH - II 18 AIZAWL EAST Aizawl (NT) RAMHLUN NORTH - III 19 AIZAWL EAST Aizawl (NT) RAMHLUN SPORT COMPLEX 20 AIZAWL EAST Aizawl (NT) RAMHLUN VENGTHAR - I 21 AIZAWL EAST Aizawl (NT) RAMHLUN VENGTHAR - II 22 AIZAWL EAST Aizawl (NT) RAMHLUN SOUTH - I 23 AIZAWL EAST Aizawl (NT) RAMHLUN SOUTH - II 24 AIZAWL EAST Aizawl (NT) RAMHLUN SOUTH - III 25 AIZAWL EAST Aizawl (NT) RAMTHAR NORTH 26 AIZAWL EAST Aizawl (NT) RAMTHAR - I 27 AIZAWL EAST Aizawl (NT) RAMTHAR - II 28 AIZAWL EAST Aizawl (NT) AIZAWL VENGLAI 29 AIZAWL EAST Aizawl (NT) RAMHLUN VENGLAI 30 AIZAWL EAST Aizawl (NT) CHANMARI EAST - I 31 AIZAWL EAST Aizawl (NT) CHANMARI EAST - II 32 AIZAWL EAST Aizawl (NT) CHANMARI WEST - I 33 AIZAWL EAST Aizawl (NT) CHANMARI WEST - II 34 AIZAWL EAST Aizawl (NT) CHANMARI WEST - III 35 AIZAWL EAST Aizawl (NT) EDENTHAR 36 AIZAWL -

Report of the Comptroller and Auditor General of India
Report of the Comptroller and Auditor General of India for the year ended 31 March 2010 (Report No. 2) GOVERNMENT OF MIZORAM TABLE OF CONTENTS Paragraph Page Preface v Overview vii CHAPTER-I PERFORMANCE REVIEWS RURAL DEVELOPMENT DEPARTMENT Border Area Development Programme 1.1 1 FOOD, CIVIL SUPPLIES AND CONSUMER AFFAIRS DEPARTMENT Public Distribution System 1.2 29 CHAPTER-II AUDIT OF TRANSACTIONS Wasteful/Temporary Misappropriation/Avoidable/Diversion/Idle Expenditure PUBLIC WORKS DEPARTMENT Wasteful expenditure 2.1 59 INDUSTRIES DEPARTMENT Temporary misappropriation/non-accountal of Government money 2.2 61 PUBLIC WORKS DEPARTMENT Loss due to undue financial aid to contractors 2.3 62 DISTRICT COUNCILS AFFAIRS DEPARTMENT Undue financial benefit to contractor 2.4 63 SOCIAL WELFARE DEPARTMENT Grant of old age pension to ineligible beneficiaries 2.5 65 LAND REVENUE AND SETTLEMENT DEPARTMENT Avoidable expenditure 2.6 67 RURAL DEVELOPMENT DEPARTMENT Unauthorised diversion of Central assistance 2.7 68 DISTRICT COUNCILS AFFAIRS DEPARTMENT Unauthorised diversion of recurring grants 2.8 69 LAND REVENUE AND SETTLEMENT DEPARTMENT Drawal of funds before actual execution of project 2.9 70 ANIMAL HUSBANDRY AND VETERINARY DEPARTMENT Idle expenditure 2.10 72 Regularity issues and other points General Follow up of Audit Reports 2.11 74 Response to audit observations and compliance thereof by the 2.12 75 Executive Audit Report (Civil) (Report No. 2) for the year ended 31 March 2010 Paragraph Page CHAPTER-III INTEGRATED AUDIT Integrated Audit of Higher and -
State/Uts Code District Codecd Block Code Town/Village Code
State/UTs_CodeDistrict_CodeCD Block_CodeTown/Village_CodeWard_Code EB_Code State/UTs_NameDistrict_NameLevel 15 284 0012 000000 0000 000000 MIZORAM Champhai CD BLOCK 15 284 0012 000000 0000 000000 MIZORAM Champhai CD BLOCK 15 284 0012 000000 0000 000000 MIZORAM Champhai CD BLOCK 15 284 0012 271289 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271290 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271291 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271292 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271293 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271294 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271295 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271296 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271297 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271298 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271299 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271300 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271301 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271302 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271303 0000 000000 MIZORAM Champhai VILLAGE 15 284 0012 271304 0000 000000 MIZORAM Champhai VILLAGE 15 284 0013 000000 0000 000000 MIZORAM Champhai CD BLOCK 15 284 0013 000000 0000 000000 MIZORAM Champhai CD BLOCK 15 284 0013 000000 0000 000000 MIZORAM Champhai CD BLOCK 15 284 0013 271305 0000 000000 MIZORAM Champhai VILLAGE 15 284 0013 271306 0000 000000 MIZORAM Champhai VILLAGE 15 284 0013 271307 0000 000000 MIZORAM Champhai VILLAGE 15 284 0013 271308 0000 000000 MIZORAM Champhai -
'The Mizoram £XTR.A
- , . ' , , -. 'The Mizoram £XTR.A. ORDINARY ,Published by Authority�' , O \' L. XXlll Aizawl, Friday, 29.4.1994, V.is.klla 9, S. E. 1916, issue No. 56_ NOTIFICATION No. LAD/VC--27/92/23/(Pt(Al, lhe 29th April. 1994. In exercise of the powers conferred under section 3 of the Lushai Hills DhstricL (Village Councils) Act, 1953 as amended from lime to time, the Go vernor of Mizoram is pleased to publish list of Villages and to fi)l'; the number of members for each suc-h village to constitute a ViJ1agc COlillcil as shown in the ANNEXURE to this otification. R. V. laimawia, COI1lIlf. & Secy. to the Govt. of Mizoram List of Village Councils. No. of Houses and Members for Village COllncil Election, 1994 51. Name of Village Council No. of No. of No. of TolAI No. hOuses seats seals No. of to be tn be Village I elecled InO\lli· Council nated members 2 --- 3 4 5 ---6 I. N. Vanlaiphai 532 7 2 9 2. Lungkawlh 90 3 I 4 3. Lungdar 'E' 416 6 2 8 4. Mualcheng 156 4 I 5 5. Sailulak 123 4 I 5 6. Khawlailung 403 6 2 8 7. Leng III 4 I 5 8. Piler 48 3 I 4 9. Lungchhu3n 90 3 I 4 10. Sialsir 46 3 I 4 II. Bawktlang 36 3 I 4 Ex-56/94 2 12. Khawbung South 327 5 2 7 13. BungzlIng 201 5 I (. 14. Zav.'lsei 66 3 I 4 15. Khuangthing 185 4 I :; 16. Buans .. 57 3 I 4 17. -

DISTRICT ELEMENTARY EDUCATION PLAN N
SARVA SHIKSHA ABHIYAN DISTRICT ELEMENTARY EDUCATION PLAN n . y '■ INDIA \ ( CHAIVIPHAI DISTRICT MIZORAM ^ "Z r'y'iw w )T 'V r • a “ ii'jc®fvx-A<rnrt.^?fi=- yt-wnaurnt.wn%rmmm *^(ru*m^mntwu m . i i i H n i * f i \\m*»wwm »^iimi»r HAP of ' cifAMPilAi hiifrffcf ’ M A n 1 p u II „ , • A'/ /^ |9<i / >y I rt t I ^ S^ltx^rA,' \ \ y n P^tnlyan r ' ' . • r^ziLW V \ r% N C- ft uaj j-ntl , ArtirA./1 I 4 \{(ktC^itKW //,!, ^ * t/ I I /< / • ,, ^ ^'enfsM fV X a e N b it fnfkvrtrtii 4»K«J ^ tJk«U «( M-rt^ ” "* * 4- Shtfi A«<aa<^a ^ •— • • •—• • • .i J hizfZttf' 6»ixi »f —» •• • •—• • • • •—' (itfftl. Clrcf*. f». oU*- c/Avy ^ . --- S'. Mti^iUdA C. Unm^taU*4 R»«.4 '~r^j: f. fi*>aM lrtn H.^r\'j I g hut^c/- Cnf^ltkJ I \ . V <7- C»AsJr4u.<iAt0 j ( t. \B \ *^^*“*7^?^''''l afmctc (S) '/ > • y A ^ ^ i , . t i » • ^i^^-^AfcnuOA'•^A.4AfcnuOA /^ •o. l\>aH tf»i I' *■ '|-*»‘" . ■ S’ V 'k t 'fJ' •■'*.;'• • I i Vfi- ' 4 ' 4i. ‘ - f*J. ,■» ' ijjuJ .J.’ <(1 Vk , ,,». M* J* ;r:^- V . j ‘ f- ' B 1 iHa it .«u’.*!# .4 4 ’.% ^ ,* . 'i*-^ ‘ *■> " 1 ■ ' '^ V " » / "i . .’ . 4* ■ ' . , t,-f* f- / i* 'h 'if' , ' . - >s ^ ' % T ’ ^ . S, i.t* 14 % 1 » . J r • « < t l , i I- . I " I %' « \ htf %" I ', l,l,l!»\i!!.S!i!.*II.< . ^ ^ “ .-I. — ^==- /»-.* * V 1 ^ : . FOREWORD DistiicI Eleiiienlary Bdiicatioii IMaii (DEIiP) (!lminpliai District, Mizoram, has been f)repare(l under the perseverance and hard work of the Kducation Staff, Chaniphai District, Mizoram. -

District Census Handbook, Aizawl, Part XIII-A & B, Series-31, Mizoram
CENSUS OF INDIA 1981 SERIES-31 MIZORAM PARTS fXIIl A & B VILLAGE & TOWN DIRECTORY VILLAGE & TOWNWISE PRIMARY CENSUS ABSTRACT AIZA.WL DISTRICT P. LALNITHAJ'\GA of the Indian Administrative Service Director of Census Operations. Mizoram CONTENTS PAGES F0reword (iii) Preface (v) Map of the District (vii) Important Slatistics (ix) Analytical Note 1-23 (i) Ce,1suS concepts of rural and urban a\eas a~d other terms namely CensJs house, ho.;s~hold, Sc':leduled Ca~tes/Scheduled Tnbes, lIterates, main worker, marginal werker, non-worker etc, (ii) Brief History of the district and the Distri(;t Census HandboJk (iii) Scope of Village Directory, T 0wn Directory Statements and Primary Census Abstract (iv) Physical Asp("~ts !Iig\lights ~:m the chan&es in the juri:;diction of the d~strict during ,the decade includmg Its boundanes and any lmportant event on GeographIc or GeophysIcal aspect (v) Mijor cll1ract~ristic, of the district p:lrticularly in relation to the economic resources . namely forestry, minerals and mining, electricity and power, land and land-use pattern, tenancy, Agriculture, animal husbandry and veterinary service, fisheries, inclll~try, trade and commerce, transport etc. (vi) Major Social and Cultural events, natural and administrative developments and misce llaneous activities of note during thc decade (vii) Brief discription of places of religio·us, historical or archaeological importance in vil1ages and places of tourist interest in the bwns of the district {viii) Brief analysis of the Village and Town Directory and Primary Census -

1 List of Health Insitution Including Hospital/Private
LIST OF HEALTH INSITUTION INCLUDING HOSPITAL/PRIVATE HOSPITAL/SUB-DISTRICT HOSPITAL/CHC/PHC/MAIN CENTRE/SUB-CENTRE AND CLINICS AIZAWL WEST DISTRICT HOSPITAL/PRIVATE PHC/UPHC MAIN CENTRE SUB-CENTRE SUB-CENTRE CLINICS CLINICS COVERED HOSPITAL/SUB- COV ERED VILLAGES VILLAGES DISTRICT HOSPITAL/ CHC HOSPITAL UPHC under 1) Aizawl S M/C 1) Republic S/C Republic 1) ITI Clinic ITI 1. Referral Hospital, NUHM 2) Upper Republic S/C Upper Republic 2) Tuikhuahtlang Clinic Tuikhuahtlang Falkawn 1. Chawlhhmun 3) Mission Veng S/C Mission Vengthlang 3) Republic Vengthlang Republic Vengthlang 2. Dist. TB Centre, 2. Hlimen 4) Kulikawn S/C Kulikawn 4) Thakthing Clinic Thakthing Aizawl Dist., 3. Lawipu 5) Venghnuai S/C Venghnuai 5) Model Veng Clinic Model Veng Falkawn 6) Salem S/C Salem Veng 6) Mission Veng Clinic Mission Veng 7) Tlangnuam S/C Tlangnuam 7) S. Hlimen Clinic S. Hlimen SUB-DISTICT Tlangnuam Vengthar 8) Samtlang Clinic Samtlang HOSPITAL 8) Melthum S/C Melthum 9) Lungleng Clinic Lungleng 1. Kulikawn Hospital Saikhamakawn 10) Damveng Clinic Damveng 9) Lungleng S/C Lungleng PRIVATE HOSPITAL 10) Hualngohmun S/C Hualngohmun 1. Aizawl Hospital, 11) Melriat S/C Melriat Khatla 2) Aizawl West 1) Zotlang S/C Zotlang 1) Kanan Clinic Kanan 2. BN Hospital, M/C 2) Vaivakawn S/C Vaivakawn 2)Rangvamual Clinic Rangvamual Kulikawn 3) Hunthar S/C Hunthar Phunchawng 3. Alpha Hospital, Kulikawn 4) Tanhril Tanhril 3) Bungkawn Vengthar Bungkawn Vengthar 4. Seventh Day Tuivamit 4) Lawipu Clinic Lawipu Hospital, Vaivakawn 5) Sakawrtuichhun S/C Sakawrtuichhun 5) Dinthar -
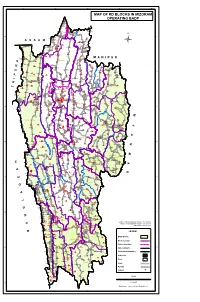
Map of Rd Blocks in Mizoram Operating Badp
92°20'0"E 92°40'0"E 93°60'0"E 93°20'0"E 93°40'0"E MAP OF RD BLOCKS IN MIZORAM Vairengte II OPERATING BADP Vairengte I Saihapui (V) Phainuam Chite Vakultui Saiphai Zokhawthiang North Chhimluang North Chawnpui Saipum Mauchar Phaisen Bilkhawthlir N 24°20'0"N 24°20'0"N Buhchang Bilkhawthlir S Chemphai North Thinglian Bukvannei I Tinghmun BuBkvIaLnKneHi IAI WTHLIR Parsenchhip Saihapui (K) Palsang Zohmun Builum Sakawrdai(Upper) Thinghlun(Lushaicherra) Hmaibiala Veng Rengtekawn Kanhmun South Chhimluang North Hlimen Khawpuar Lower Sakawrdai Luimawi KOLASIB N.Khawdungsei Vaitin Pangbalkawn Hriphaw Luakchhuah Thingsat Vervek E.Damdiai Bungthuam Bairabi New_Vervek Meidum North Thingdawl Thingthelh Lungsum Borai Saikhawthlir Rastali Dilzau H Thuampui(Zawlnuam) Suarhliap R Vengpuh i(Zawlnuam) i Chuhvel Sethawn a k DARLAWN g THINGDAWL Ratu n a Zamuang Kananthar L Bualpui Bukpui Zawlpui Damdiai Sunhluchhip Lungmawi Rengdil N.Khawlek Hortoki Sailutar Sihthiang R North Kawnpui I i R Daido a Vawngawnzo l Vanbawng v i Tlangkhang Kawnpui w u a T T v Mualvum North Chaltlang N.Serzawl i u u Chiahpui i N.E.Tlangnuam Khawkawn s T Darlawn a 24°60'0"N 24°60'0"N Lamherh R Kawrthah Khawlian Mimbung K Sarali North Sabual Sawleng Chilui Zanlawn N.E.Khawdungsei Saitlaw ZAWLNUAM Lungmuat Hrianghmun SuangpuilaPwnHULLEN Vengthar Tumpanglui Teikhang Venghlun Chhanchhuahna kepran Khamrang Tuidam Bazar Veng Nisapui MAMIT Phaizau Phuaibuang Liandophai(Bawngva) E.Phaileng Serkhan Luangpawn Mualkhang Darlak West Serzawl Pehlawn Zawngin Sotapa veng Sentlang T l Ngopa a Lungdai -

DELIMITATION COMMISSION of INDIA NIRVACHAN SADAN, ASHOKA ROAD, NEW DELID-110001 SHANGARARAM Lel
VOL - XXXIV Aizawl, Friday, 27.5.2005 Jyaistha 6, S.E. 1927, Issue No.1 07 DELIMITATION COMMISSION OF INDIA NIRVACHAN SADAN, ASHOKA ROAD, NEW DELID-110001 SHANGARARAM lEL. 011-23052005 SECRETARY FAX 011-23052006 NO. 282/MIZ/2005 Dated: 27th May, 2005 6 Jyeshtha, 1927 (Saka) NOTIFICATION Inpursuanceof Sub-Section(1) ofSection 10oftheDelimitationAct,2002 (33 of2002), the following OrdermadebytheDelimitation Commission underSub-Section (2)ofSection 9read withSub-Section (2)ofSection4oftheAct,inrespectofthedelimitation ofParliamentary and Assembly Constituencies intheStateof Mizoramishereby published. ORDER NO. 10 WHEREAS, inpursuanceofSections8and4 oftheDelimitationAct, 2002 (33 of2002), as amended bytheDelimitation (Amendment) Act,2003 (3of2004),theDelimitation Commission has, by its Order No. 7 dated 13th April, 2005, published in the extraordinary issue ofthe Gazette ofIndiaandthe Mizorarn StateGazetteon 13thApril,2005,determined - (i) thetotal numberofseats in the House ofthe Peopleto be allocatedto the StateofMizoram as one (I ) which shallbereserved fortheScheduled Tribes, and(ii) thetotal numberofseatstobeassigned, . to theLegislative Assemblyofthe Stateas forty (40)ofwhich,no seatshallbereserved forthe Scheduled Castesandthirtynine(39)seatsfortheScheduled Tribes; and WHEREAS, in pursuanceof Sub-Section (l)ofSection5, read with subSection(l) of Section9 ofthe said Act, the DelimitationCommissionassociatedwith itself the Associate Members fromtheStatewiththeproceedings forthedelimitation ofParliamentary andAssembly Constituencies intheState;and