Impact of Transjugular Intrahepatic Portosystemic Shunt Creation on The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Salvage Cisterna Chyli and Thoracic Duct Glue Embolization in 2 Dogs with Recurrent Idiopathic Chylothorax
Case Report J Vet Intern Med 2014;28:672–677 Salvage Cisterna Chyli and Thoracic Duct Glue Embolization in 2 Dogs with Recurrent Idiopathic Chylothorax D.C. Clendaniel, C. Weisse, W.T.N. Culp, A. Berent, and J.A. Solomon Key words: Chylous; Fluoroscopy; Interventional Radiology; Pleural Effusion; Thoracocentesis. Case Report Abbreviations: Case 1 PO administered by mouth 4-year-old male castrated English Mastiff weigh- PRN administered as needed Aing 72 kg (158 lb) was evaluated for acute dysp- nea after a 1-week history of restlessness, episodes of increased respiratory effort, and inappetance. The dog The sample was too lipemic to obtain an accurate total was otherwise healthy and received seasonal heart- protein concentration. No microorganisms or atypical worm preventative medication and routine vaccina- cells were identified. Comparison of the fluid triglycer- tions. ide concentration with the serum triglyceride concen- On initial examination, the dog was bright, alert, tration (57 mg/dL; reference interval 50–150 mg/dL), responsive, and had a body condition score of 5/9. combined with cytologic findings, was consistent with The body temperature was 101.8°F (38.7°C), and the chylous effusion. heart rate was 150 beats/min. There was an increased Thoracic radiography revealed a small hyperlucent respiratory effort during both inspiration and expira- region in the caudodorsal thorax, suggesting mild tion with an abdominal component. Thoracic ausculta- pneumothorax presumably secondary to recent tho- tion revealed decreased lung sounds in the ventral lung racocentesis. No evidence of valvular or myocardial fields. Heart sounds were of normal rhythm, but muf- disease was observed during echocardiogram evalua- fled. -

The Cisterna Chyli and Thoracic Duct in Pigs (Sus Scrofa Domestica)
Original Paper Veterinarni Medicina, 55, 2010 (1): 30–34 The cisterna chyli and thoracic duct in pigs (Sus scrofa domestica) M. Duras Gomercic1, T. Trbojevic Vukicevic1, T. Gomercic1, A. Galov2, T. Fruk3, H. Gomercic1 1Faculty of Veterinary Medicine, University of Zagreb, Croatia 2Faculty of Science, University of Zagreb, Croatia 3Veterinary Station of Varazdin, Croatia ABSTRACT: Anatomical variations of the thoracic duct course are common in humans and domestic animals. They are important in thoracic surgery and in application of surgical techniques in experimental animals. The pig is a frequently used animal model due to numerous similarities between human and porcine anatomy and physiology. We revealed the position of the cisterna chyli, and the origin, course and termination of the thoracic duct by fine dissection on fifteen Yorkshire pig carcasses. The pigs were 2.5 months old with a body mass range from 10 to 15 kg. In this study we present our macroscopic observations. The cisterna chyli and thoracic duct had a common position, form and course in ten (67%) specimens. Anatomical variations of the precardiac course of the thoracic duct were observed in five animals (33%). Knowledge of these anatomical features should enhance the use of swine as an experimental model. Keywords: anatomy; lymphatic system; swine The thoracic duct is the chief collecting vessel due to numerous similarities between human and of the lymphatic system (Sisson and Grossman, porcine anatomy and physiology. The use of pigs 1956). It conveys lymph from the cisterna chyli to for teaching purposes in medicine and surgery has the venous angle (Vollmerhaus, 1981). The cisterna increased greatly in recent years, because they are chyli receives lymph from the abdomen, pelvis and tractable, readily available from commercial sup- hindlimbs. -

Board Review for Anatomy
Board Review for Anatomy John A. McNulty, Ph.D. Spring, 2005 . LOYOLA UNIVERSITY CHICAGO Stritch School of Medicine Key Skeletal landmarks • Head - mastoid process, angle of mandible, occipital protuberance • Neck – thyroid cartilage, cricoid cartilage • Thorax - jugular notch, sternal angle, xiphoid process, coracoid process, costal arch • Back - vertebra prominence, scapular spine (acromion), iliac crest • UE – epicondyles, styloid processes, carpal bones. • Pelvis – ant. sup. iliac spine, pubic tubercle • LE – head of fibula, malleoli, tarsal bones Key vertebral levels • C2 - angle of mandible • C4 - thyroid notch • C6 - cricoid cartilage - esophagus, trachea begin • C7 - vertebra prominence • T2 - jugular notch; scapular spine • T4/5 - sternal angle - rib 2 articulates, trachea divides • T9 - xiphisternum • L1/L2 - pancreas; spinal cord ends. • L4 - iliac crest; umbilicus; aorta divides • S1 - sacral promontory Upper limb nerve lesions Recall that any muscle that crosses a joint, acts on that joint. Also recall that muscles innervated by individual nerves within compartments tend to have similar actions. • Long thoracic n. - “winged” scapula. • Upper trunk (C5,C6) - Erb Duchenne - shoulder rotators, musculocutaneous • Lower trunk (C8, T1) - Klumpke’s - ulnar nerve (interossei muscle) • Radial nerve – (Saturday night palsy) - wrist drop • Median nerve (recurrent median) – thenar compartment - thumb • Ulnar nerve - interossei muscles. Lower limb nerve lesions Review actions of the various compartments. • Lumbosacral lesions - usually -

Lymph and Lymphatic Vessels
Cardiovascular System LYMPH AND LYMPHATIC VESSELS Venous system Arterial system Large veins Heart (capacitance vessels) Elastic arteries Large (conducting lymphatic vessels) vessels Lymph node Muscular arteries (distributing Lymphatic vessels) system Small veins (capacitance Arteriovenous vessels) anastomosis Lymphatic Sinusoid capillary Arterioles (resistance vessels) Postcapillary Terminal arteriole venule Metarteriole Thoroughfare Capillaries Precapillary sphincter channel (exchange vessels) Copyright © 2010 Pearson Education, Inc. Figure 19.2 Regional Internal jugular vein lymph nodes: Cervical nodes Entrance of right lymphatic duct into vein Entrance of thoracic duct into vein Axillary nodes Thoracic duct Cisterna chyli Aorta Inguinal nodes Lymphatic collecting vessels Drained by the right lymphatic duct Drained by the thoracic duct (a) General distribution of lymphatic collecting vessels and regional lymph nodes. Figure 20.2a Lymphatic System Outflow of fluid slightly exceeds return Consists of three parts 1. A network of lymphatic vessels carrying lymph 1. Transports fluid back to CV system 2. Lymph nodes 1. Filter the fluid within the vessels 3. Lymphoid organs 1. Participate in disease prevention Lymphatic System Functions 1. Returns interstitial fluid and leaked plasma proteins back to the blood 2. Disease surveillance 3. Lipid transport from intestine via lacteals Venous system Arterial system Heart Lymphatic system: Lymph duct Lymph trunk Lymph node Lymphatic collecting vessels, with valves Tissue fluid Blood Lymphatic capillaries Tissue cell capillary Blood Lymphatic capillaries capillaries (a) Structural relationship between a capillary bed of the blood vascular system and lymphatic capillaries. Filaments anchored to connective tissue Endothelial cell Flaplike minivalve Fibroblast in loose connective tissue (b) Lymphatic capillaries are blind-ended tubes in which adjacent endothelial cells overlap each other, forming flaplike minivalves. -

Dilated Cisternae Chyli. a Sign of Uncompensated Cirrhosis at MR Imaging
Thomas Jefferson University Jefferson Digital Commons Department of Radiology Faculty Papers Department of Radiology January 2008 Dilated cisternae chyli. A sign of uncompensated cirrhosis at MR imaging Sachit K. Verma Thomas Jefferson University Donald G. Mitchell Thomas Jefferson University Diane Bergin Thomas Jefferson University Yulia Lakhman Thomas Jefferson University FAmyollow A thisustin and additional works at: https://jdc.jefferson.edu/radiologyfp Thomas Jefferson University Part of the Radiology Commons Let us know how access to this document benefits ouy See next page for additional authors Recommended Citation Verma, Sachit K.; Mitchell, Donald G.; Bergin, Diane; Lakhman, Yulia; Austin, Amy; Verma, Manisha; Assis, David; Herrine, Steven K.; and Parker, Laurence, "Dilated cisternae chyli. A sign of uncompensated cirrhosis at MR imaging" (2008). Department of Radiology Faculty Papers. Paper 4. https://jdc.jefferson.edu/radiologyfp/4 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Department of Radiology Faculty Papers by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. Authors Sachit K. Verma, Donald G. Mitchell, Diane Bergin, Yulia Lakhman, Amy Austin, Manisha Verma, David Assis, Steven K. -

Patterns of Lymphatic Drainage in the Adultlaboratory
J. Anat. (1971), 109, 3, pp. 369-383 369 With 11 figures Printed in Great Britain Patterns of lymphatic drainage in the adult laboratory rat NICHOLAS L. TILNEY Department of Surgery, Peter Bent Brigham Hospital, and Harvard Medical School, Boston, Massachusetts (Accepted 27 April 1971) INTRODUCTION This study was undertaken to define and elucidate patterns of lymphatic drainage in the adult laboratory rat. The incentive for the work arose from investigations into the role of regional lymphatics in the sensitization of the host by skin allografts. It has become clear that the response of rats to antigens, investigated increasingly in the available inbred strains, requires an accurate knowledge of lymphoid anatomy and lymphatic drainage routes. Examinations of the lymphatics of specific body areas of the rat have appeared sporadically in the literature, but descriptions of regional drainage patterns, especially of peripheral sites, are unavailable. Previous investigations by Job (1919), Greene (1935) and Sanders & Florey (1940) have con- centrated primarily upon the location of the lymphoid tissues. Miotti (1965) has stressed visceral drainage, and Higgins (1925) has described the lymphatic system of the newborn rat. A more complete definition of both somatic and visceral lymphatic routes is presented. MATERIALS AND METHODS One hundred and thirty normal adult rats of both sexes, each weighing between 150 and 300 g, were studied. The animals came from five strains: each inbred - Oxford strains of the albino (AO), hooded (HO), agouti (DA), and F1 hybrid of the HO and DA strains - and 'stock' animals from a closed outbred albino colony. Under ether anaesthesia, the site for cutaneous injection was clipped or a serous cavity entered for visceral injection. -

The Lymphatic System of the Human Body
THE LYMPHATIC SYSTEM OF THE HUMAN BODY We love your lymphatics We want you to love them too! THERAPIST QUALIFICATIONS VODDER ACADEMY IN AUSTRIAN TYROL Recuperating cancer patients attend the clinic for two weeks, soon after their medical treatment, for post-operative treatments, early preventative treatment for Oedema and to learn how to self-treat themselves at home. OUR SERVICES HOW MUCH DOES IT COST? TREATMENTS REBATES • Physiotherapy $115/hr • Private Health rebates on Physio/OT/RMT • Occupational Therapy $115/hr • CHP (Chronic Health Plan) up to 5/pa Medicare subsidised treatments for Physio/OT • Remedial/Pregnancy Massage $85/hr for $52.95 (including Sozo) • Sozo Testing $55/30 mins • DVA – fully subsidised up to 20/pa • Garment Fitting $55/30 mins • ACAT – fully subsidised up to level of cover • ICWA (Insurance Commission WA) ROLE OF THE LYMPHATIC SYSTEM • Sewerage System of the body • Fluids - uptake and clearance that can’t get back into th blood vessels • Absorption of fats (long chain fatty acids) • Maintains blood volume • Immune response WASTE MAINTENANCE • Collects & drains: 2-4L Lymph per day. • 100mls lymph drain from each arm daily = about a ½ cup of tea. • 200-300mls drain from each leg daily = about 1½ cups of tea. • Works at about 20% of capacity. LYMPH SYSTEM • Superficial and deep • 80% above the muscle 20% deep • 60% cervical • Lymph from the legs, groin, abdomen and left chest, arm and neck drain to left terminus • Lymph form right chest, arm and side of head to the right terminus IT ALL STARTS IN THE SKIN! ILV & ANCHORING FILAMENTS THE FACTORIES – LYMPH NODES • Holds invaders (pathogens) to identify! • Immune response factory • Waste recycling plant • Returns H2O back to blood • Stores insolubles – Ink! • We can’t make new nodes THORACIC DUCT • Thoracic duct 38-45cms long & 5mm in diameter, largest lymphatic vessel = Super Highway! • Cisterna chyli is a dilated lymphatic sac that represents origin of thoracic duct = Vacuum Cleaner! • Clears the whole of the lower part of the body and upper left quadrant. -

Thoracic Duct Embolization Using Direct Hybrid Angiography
Published online: 2021-03-26 Case Report Thoracic Duct Embolization Using Direct Hybrid Angiography/Computed Tomography‑Guided Cisterna Chyli Access for the Treatment of Chylous Leak Secondary to Partial Glossectomy and Neck Dissection Abstract Karan Nijhawan, Thoracic duct embolization (TDE) is a minimally invasive alternative to surgery for the treatment of Divya Kumari, postoperative chylous leaks that fail conventional medical management. TDE can be a technically Brian Funaki, challenging procedure that requires real-time image guidance to visualize the thoracic duct. This case report describes using hybrid angiography/computed tomography technology to perform TDE through Osman Ahmed direct access of the cisterna chyli, potentially eliminating the need for intranodal lymphangiography, Department of Radiology, and reducing procedure length. Section of Vascular and Interventional Radiology, Keywords: Chylous leak, cisterna chyli, hybrid angiography/computed tomography, thoracic duct University of Chicago Medicine, Chicago, IL, USA embolization Introduction complicated by a chylous leak for which a surgical drain was placed in the left Thoracic duct embolization (TDE) neck. Postoperatively, chylous output is a technically challenging and time was >1000 ml/day for 10 days with intensive procedure that relies on the maximum daily chylous output of 1850 ml successful cannulation of the TD for despite optimal medical management with direct visualization of chylous leakage and total parenteral nutrition and octreotide. endolymphatic into inguinal lymph nodes Triglycerides were elevated at 285 mg/ followed by a variable waiting period to dL (normal range: 30–149 mg/dL). TDE opacify the cisterna chyli (CC) to facilitate was performed in a room equipped with an the fluoroscopic puncture. For intranodal Angio-CT system (Infinix-I 4DCT, Canon, lymphangiography, mean transit time Tustin, CA) using local anesthesia and until the visualization of target lymphatic conscious sedation. -

ANATOMICAL VARIATIONS of the HUMAN THORACIC DUCT Bapuji
International Journal of Anatomy and Research, Int J Anat Res 2018, Vol 6(4.2):5861-68. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2018.362 ANATOMICAL VARIATIONS OF THE HUMAN THORACIC DUCT Bapuji. P 1, Srinivasarao Yalakurthi *2, Thirupathirao Vishnumukkala 3, Swayam Jothi Dorai Raj 4. 1 Professor & Head, Department of Anatomy, ASRAM Medical college, Eluru, India. *2 Assistant Professor, Department of Anatomy, ASRAM Medical College, Eluru, India. 3 Lecturer of Anatomy, PU-RCSI School of Medicine, Perdana University, Malaysia. 4 Professor & Head, Department of Anatomy, SSSMCRI, Nellikuppam, India. ABSTRACT Background and Aims: The thoracic duct is the major lymphatic duct in the human body, the variations in the origin, course and termination of the thoracic duct are of great clinical importance during surgeries related to the upper abdomen, posterior mediastinum and in cervical region, but still now a detailed study had not been done in Andhra Pradesh state, it was decided to undertake this present study. Isolation of the thoracic duct and tracing the origin, course and termination were done to know more about it than already documented and thereby hoping to add more information to guide the radiologists and operating surgeons. Materials and methods: A total number of 45 cadavers were studied, of these 15 were female and 30 were male cadavers. The material consisted of adult cadavers between the ages of 42-81 from the dissection halls of department of anatomy of 3 different medical colleges in costal Andhra Pradesh. Results: The observations of the formation, course, length, vertebral levels, types of cisterna chili, and variations in the termination in cervical region are documented in this study. -
Endocrine System: Overview
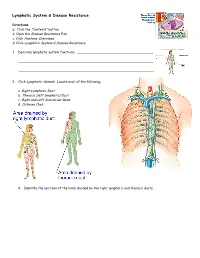
Lymphatic System & Disease Resistance Directions: a. Click the “Contents” button. b. Open the Disease Resistance File. c. Click Anatomy Overviews. d. Click Lymphatic System & Disease Resistance. 1. Describe lymphatic system functions. 2. Click Lymphatic Vessels. Locate each of the following: a. Right Lymphatic Duct b. Thoracic (left lymphatic) Duct c. Right and Left Subclavian Veins d. Cisterna Chyli d. Identify the sections of the body drained by the right lymphatic and thoracic ducts. e. Determine the direction of blood and lymph movement between arterioles, blood and lymph capillaries, and venules. f. Describe the lymphatic system role with regard to lipids and lipid- soluble vitamins. 3. Return to the main Lymphatic System page and click the Thymus Gland. a. Identify the thymus gland. Note its location relative to the heart (covered by parietal pericardium in this view). b. What are the thymus gland functions? 4. Return to the main Lymphatic System page and click the Spleen and Lymph Node. Identify each of the following: a. Afferent Lymphatic Vessels b. Efferent Lymphatic Vessels c. What is the function of the macrophages within the lymph nodes? d. Identify spleen functions. 5. Return to the main Lymphatic System page and click the T Lymphocytes. a. Some activated cytotoxic T cells produce perforin. What is the function of perforin? 6. b. Some activated cytotoxic T cells produce Lymphotoxin. What is the function of lymphotoxin? 7. Return to the main Lymphatic System page and click the B Lymphocytes. Activated B Lymphocytes are transformed into plasma cells that produce antibodies. Name five ways antibodies can destroy foreign cells and substances. -

Receives Lymph Drainage from Digestive Organs
Venous Arterial system system Heart Lymph duct Lymph trunk Lymph node Lymphatic system Lymphatic collecting vessels, with valves Lymph capillary Tissue fluid (becomes lymph) Blood Loose connective capillaries tissue around capillaries © 2018 Pearson Education, Inc. 1 Tissue fluid Tissue cell Lymphatic capillary Blood capillaries (a) Arteriole Venule © 2018 Pearson Education, Inc. 2 Fibroblast in loose connective tissue Flaplike minivalve Filaments anchored to Endothelial connective cell tissue (b) © 2018 Pearson Education, Inc. 3 Entrance of right Regional lymphatic duct into right lymph nodes: subclavian vein Cervical Internal jugular vein nodes Thoracic duct entry into left subclavian vein Axillary nodes Thoracic duct Aorta Spleen Cisterna chyli (receives lymph drainage from Inguinal digestive organs) nodes Lymphatics KEY: Drained by the right lymphatic duct Drained by the thoracic duct © 2018 Pearson Education, Inc. 4 Germinal center in Afferent follicle Capsule lymphatic vessels Subcapsular sinus Trabecula Afferent Efferent lymphatic lymphatic vessels vessels Hilum Cortex Medullary sinus Follicle Medullary cord © 2018 Pearson Education, Inc. 5 Tonsils (in pharyngeal region) Thymus (in thorax; most active during youth) Spleen (curves around left side of stomach) Peyer’s patches (in intestine) Appendix © 2018 Pearson Education, Inc. 6 The Immune System Adaptive (specific) defense Innate (nonspecific) defense mechanisms mechanisms First line of defense Second line of defense Third line of defense • Skin • Phagocytic cells • Lymphocytes -

Lymphatic System
OUTLINE 24.1 Functions of the Lymphatic System 725 24.2 Lymph and Lymph Vessels 726 24 24.2a Lymphatic Capillaries 726 24.2b Lymphatic Vessels 726 24.2c Lymphatic Trunks 727 24.2d Lymphatic Ducts 727 Lymphatic 24.3 Lymphatic Cells 729 24.3a Types and Functions of Lymphocytes 729 24.3b Lymphopoiesis 734 24.4 Lymphatic Structures 735 System 24.4a Lymphatic Nodules 735 24.4b Lymphatic Organs 736 24.5 Aging and the Lymphatic System 741 24.6 Development of the Lymphatic System 741 MODULE 10: LYMPHATIC SYSTEM mck78097_ch24_724-746.indd 724 2/25/11 2:16 PM Chapter Twenty-Four Lymphatic System 725 e have seen in chapters 21–23 how the cardiovascular system W transports blood throughout the body, where it exchanges gases and nutrients with the tissues. Another body system, called the lymphatic system, aids the cardiovascular system by transporting excess interstitial fluid through lymph vessels assisting in maintain- ing fluid homeostasis. Once this fluid enters the vessels, the fluid is Tonsils renamed lymph. Along the way, lymph is filtered and checked for Cervical lymph nodes foreign or pathologic material, such as bacteria and cancer cells. Right lymphatic duct Lymphatic structures contain certain cells that initiate an immune Axillary response to abnormal materials. Without the primary immune Thymus response by the lymphatic system, the body would be unable to fight lymph nodes infection and keep itself healthy. In this chapter we examine the lymph vessels, lymphatic Thoracic duct structures, and lymphatic organs of the body, and learn how each Spleen Cisterna chyli of these components plays an important role in keeping us healthy.