Studies on the Formation of Nitrosamines (VI)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cyanide Poisoning and How to Treat It Using CYANOKIT (Hydroxocobalamin for Injection) 5G
Cyanide Poisoning and How to Treat It Using CYANOKIT (hydroxocobalamin for injection) 5g 1. CYANOKIT (single 5-g vial) [package insert]. Columbia, MD: Meridian Medical Technologies, Inc.; 2011. Please see Important Safety Information on slides 3-4 and full Prescribing Information for CYANOKIT starting on slide 33. CYANOKIT is a registered trademark of SERB Sarl, licensed by Meridian Medical Technologies, Inc., a Pfizer company. Copyright © 2015 Meridian Medical Technologies, Inc., a Pfizer company. All rights reserved. CYK783109-01 November/2015. Indication and Important Safety Information……………………………………………………………………………….………..…..3 . Identifying Cyanide Poisoning……………………………………………………………………………………………………………….…………….….5 . How CYANOKIT (hydroxocobalamin for injection) Works……………………………………………………………….12 . The Specifics of CYANOKIT…………………………………………………………………………………………………………………………….………17 . Administering CYANOKIT………………………………………………………………………………………………………………………………..……….21 . Storage and Disposal of CYANOKIT…................................................................................................................................26 . Grant Information for CYANOKIT……………………………………………………………………………………………………………………....30 . Full Prescribing Information………………………………………………………………………………………………….………………………………33 Please see Important Safety Information on slides 3-4 and full Prescribing Information for CYANOKIT starting on slide 33. CYANOKIT (hydroxocobalamin for injection) 5 g for intravenous infusion is indicated for the treatment of known or suspected cyanide poisoning. -

Evaluation of Neuropharmacological Activity of Hydroalcoholic Extract of Fruits of Trapa Bispinosa in Laboratory Animals
International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 2, Suppl 2, 2010 Research Article EVALUATION OF NEUROPHARMACOLOGICAL ACTIVITY OF HYDROALCOHOLIC EXTRACT OF FRUITS OF TRAPA BISPINOSA IN LABORATORY ANIMALS VYAWAHARE N. S.a AND AMBIKAR D. B.* b aDepartment of Pharmacology, AISSMS College of Pharmacy, Kennedy Road, Pune 411 001, India. bDepartment of Pharmacology, Marathwada Mitra Mandals College of Pharmacy, Thergaon, Pune 411033, India. Email: [email protected] Received: 26 Dec 2009, Revised and Accepted:19 Jan 2010 ABSTRACT The present investigation deals with evaluation of neuropharmacological activity of different doses (100, 250, 500mg/kg, p.o.) of hydroalcoholic extract of Trapa bispinosa (TB) in laboratory animals. The effects of extract on various parameters like motor coordination, spontaneous locomotor activity, object recognition, transfer latency, anxiolytic activity, analgesic activity, sodium nitrite induced respiratory arrest and hypoxic stress etc was studied. The TB (250 & 500mg/kg) found to decrease time required to occupy the central platform (transfer latency) in the elevated plus maze and to increase discrimination index in the object recognition test, indicating nootropic activity. TB (250 and 500mg/kg) showed significant increase in reaction time in hot plate analgesic activity. Moreover it also showed significant reduction in spontaneous locomotor activity and latency to death in sodium nitrite induced respiratory arrest. To conclude hydroalcoholic extract of TB possesses significant facilitation of learning and memory which may be due to enhanced cholinergic function. TB also showed significant analgesic activity. Key words: Trapa bispinosa, Nootropic, Analgesic. Chemicals and drugs INTRODUCTION Sodium nitrate was purchased from Loba chemicals, Mumbai. Medicinal herbs constitute the cornerstone of traditional medicinal Diazepam injection, pentazocin injection, piracetam syrup, was practice worldwide. -

Letter to the Editor Effect of N-Acetylcysteine And
Indian J Physiol Pharmacol 1999; 43 (4): 518-520 LETTER TO THE EDITOR EFFECT OF N-ACETYLCYSTEINE AND SODIUM NITRITE ON MYOCARDIAL REPERFUSION INJURY Sir, (Received on May 22, 1999) Results of experimental studies suggest India). Heart was suspended in pericardial the involvement of oxygen free radicals cradle after left sided thoracotomy at fourth (OFR) in myocardial reperfusion injury 0, intercostal space. A polythene catheter 2). Ischemia is known to deplete endogenous (1.5 mm inner diameter) was placed in the antioxidants like superoxide dismutase, left ventricle through apex t~ [ecord glutathione, catalase 0, 2) and makes ventricular pressure changes on Grass 16 myocardial, cell vulnerable to oxygen free channel polygraph (Model 79D, USA) by radical (OFR) induced damage 0, 2). OFR using Gould pressure transducer. Heart rate can damage endothelial cells at the time of and ST segment changes were monitored in reperfusion (3). Endothelial cells synthesise standard limb leads on BPL (India) and form nitric oxide which is endothelium, electrocardiograph. Another two catheters derived relaxation factor (4). Acidified were placed in the left atrium for infusion sodium nitrite (Na Oz) at pH-2 forms nitric of saline, drug or acidified N aNOz' Left oxide (5) which can inactivate superoxide anterior descending (LAD) coronary artery radicals (5, 6). N-acetylcysteine (NAC) is was dissected free above first diagonal known to replenish glutathione stores, branch and below the origin of left increase superoxide dismutase activity, circumflex artery. Duration of LAD coronary scanvenge hydroxyl radicals and interfere artery occlusion was 90-min. Following with autocatalytic lipid peroxidation (7, 8). completion of occlusion, reperfusion of the In our previous studies we have shown the myocardium was initiated by removing the individual effects of NAC and NaNOz on vessel occludeI'. -

Long-Term N-Acetylcysteine and L-Arginine Administration Reduces Endothelial Activation and Systolic Blood Pressure in Hypertensive Patients with Type 2 Diabetes
Emerging Treatments and Technologies ORIGINAL ARTICLE Long-Term N-Acetylcysteine and L-Arginine Administration Reduces Endothelial Activation and Systolic Blood Pressure in Hypertensive Patients With Type 2 Diabetes 1 1 VALENTINO MARTINA, MD PAOLA MASSARENTI, MD ardiovascular complications repre- 1 1 ANDI MASHA, MD FABIO SETTANNI, PHD sent 80% of the causes of death in 1 2 VALENTINA RAMELLA GIGLIARDI, MD LARA DELLA CASA, PHD 1 patients with type 2 diabetes. Several OREDANA ROCATO MD 2 C L B , STEFANIA BERGAMINI, PHD causes may explain this mortality excess. 3 2 ENZO MANZATO, MD, PHD NNA ANNONE MD, PHD 1 A I , Among these, the decreased availability of ARRIGO BERCHIO, MD nitric oxide (NO) has increasingly gained credit. In fact, reduced NO availability has been demonstrated not only in diabe- OBJECTIVE — Reactive oxygen and nitric oxide (NO) have recently been considered to be tes (1) but also in other diseases, such as involved in the cardiovascular complications of patients with type 2 diabetes, as NO is thought atherosclerosis and hypertension, known to lose its beneficial physiological effects in the presence of oxygen radicals. For this reason, we to be associated with increased mortality tested the effects of L-arginine (ARG) and N-acetylcysteine (NAC) administration in increasing due to cardiovascular causes (2,3). NO is NO bioavailability by reducing free radical formation. crucial for regulating the vascular tone RESEARCH DESIGN AND METHODS — A double-blind study was performed on 24 and maintaining the intrinsic thrombore- male patients with type 2 diabetes and hypertension divided into two groups of 12 patients that sistant and atheroprotective properties of randomly received either an oral supplementation of placebo or NAC ϩ ARG for 6 months. -

Sodium Nitrite and Sodium Thiosulfate
PATIENT & CAREGIVER EDUCATION Sodium Nitrite and Sodium Thiosulfate This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Nithiodote Warning This drug may cause low blood pressure and a red blood cell problem called methemoglobinemia. These may be life-threatening. This drug is only for use when cyanide poisoning is life-threatening. This drug must be used with care if it is not known if cyanide poisoning has happened. Talk with the doctor. Tell the doctor if your child has inhaled a lot of smoke or if your child has any of these health problems: Anemia, heart problems, lack of a certain enzyme called congenital methemoglobin reductase deficiency, or lung problems. What is this drug used for? It is used to treat cyanide poisoning. What do I need to tell the doctor BEFORE my child takes this drug? If your child is allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell the doctor about the allergy and what signs your child had. Sodium Nitrite and Sodium Thiosulfate 1/6 If your child is breast-feeding a baby: Be sure your child does not breast-feed a baby while taking this drug. This drug may interact with other drugs or health problems. Tell the doctor and pharmacist about all of your child’s drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe to give this drug with all of your child’s other drugs and health problems. -

Annex 1: Standard Operation Procedures (SOP) for Environmental Monitoring
Project Completion Report Annex Annex 1: Standard Operation Procedures (SOP) For Environmental Monitoring 1.5 Air Quality Nippon Koei Co., Ltd. The Capacity Development of Environmental Monitoring at Directorates Standard Operation Procedure (SOP)-01 Sampling and Concentration Calculation Procedure of Nitrogen Oxide (NOX) Air Quality Analysis Date of preparing SOP Work Sheet (Air quality) Measuring substance Nitrogen Oxide (NOX) Measurement method Saltzman Reaction Method 1- Measurement flowchart and main items (1) Preparation of equipments and tools (Reference) ① Selection of absorption tube. ② Preparation of the handy sampler. (2) Adjustment of the reagents ① Making the solution of 0.1% N-(1-Naphthyl) ethylenediamine dihydrochloride (hereafter, it is called NEDA solution) ② Making the absorption solution ③ Making the standard undiluted solution NaNO2 (2.03 g/L) ④ Making the standard solution NaNO2 (0.0203 g/L) ⑤ Making oxidation solution (3) Carrying out the measurement ① Establishment of the sampling locations ② Collecting the samples (sampling) ③ Making sampling solution for the analysis purpose (4) Making the analysis ① Measuring the absorbance of the collected samples ② Measuring the absorbance of the standard solution NaNO2 (5) Calculating the concentration ① Drawing the calibration curve ② Calculating the concentration of NO2 ③ Calculating the concentration of NO - 1 - 2.Measurement principle If we put the air sample which contains NO2 in an absorption coloring solution (N-(1-Naphthyl) ethylenediamine dihydrochloride, Sulfanilic acid, and Acetic acid mixture solution), we will get an orange-red azo-dye (dyestuff) that is proportional to NO2 amount. By measuring the absorbance of the coloring solution we can calculate NO2 concentration. Because NO does not react with the absorption coloring solution, it is oxidized to NO2 by using potassium permanganate (after adding sulfuric acid), then we can measure it by the same method mentioned-above. -

Effect of Sodium Nitrite, Sodium Erythorbate and Organic Acid Salts on Germination and Outgrowth of Clostridium Perfringens Spores in Ham During Abusive Cooling
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations, Theses, & Student Research in Food Science and Technology Food Science and Technology Department Fall 9-19-2011 Effect of Sodium Nitrite, Sodium Erythorbate and Organic Acid Salts on Germination and Outgrowth of Clostridium perfringens Spores in Ham during Abusive Cooling Mauricio A. Redondo University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/foodscidiss Part of the Food Chemistry Commons, Food Microbiology Commons, and the Food Processing Commons Redondo, Mauricio A., "Effect of Sodium Nitrite, Sodium Erythorbate and Organic Acid Salts on Germination and Outgrowth of Clostridium perfringens Spores in Ham during Abusive Cooling" (2011). Dissertations, Theses, & Student Research in Food Science and Technology. 18. https://digitalcommons.unl.edu/foodscidiss/18 This Article is brought to you for free and open access by the Food Science and Technology Department at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Dissertations, Theses, & Student Research in Food Science and Technology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. EFFECT OF SODIUM NITRITE, SODIUM ERYTHORBATE AND ORGANIC ACID SALTS ON GERMINATION AND OUTGROWTH OF CLOSTRIDIUM PERFRINGENS SPORES IN HAM DURING ABUSIVE COOLING By Mauricio Redondo-Solano A THESIS Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Master of Science Major: Food Science and Technology Under the supervision of Professor Harshavardhan Thippareddi Lincoln, Nebraska September, 2011 EFFECT OF SODIUM NITRITE, SODIUM ERYTHORBATE AND ORGANIC ACID SALTS ON GERMINATION AND OUTGROWTH OF CLOSTRIDIUM PERFRINGENS SPORES IN HAM DURING ABUSIVE COOLING Mauricio Redondo-Solano, M. -

Ingestions, Intoxications, and the Critically Ill Child Poisoning in Children
Ingestions, Intoxications, and the Critically Ill Child Poisoning in Children • 1 million cases of exposure to toxins in children younger than 6 years reported in the U.S. In 1993 • estimated that another 1 million exposures to toxins not reported • 1% have moderate or major life-threatening symptoms • 60-100 deaths annually in the U.S Poisoning in Children Less Than 5 Years Old • accounts for 85-90% of pediatric poisoning • is generally accidental • secondary to exploratory behavior and lack of supervision • tend to involve single agent ingestions Poisoning in Children Over 5 Years Old • accounts for 10-15% of pediatric poisoning • is generally intentional • secondary to suicide attempts or gestures, or to intoxications and inadvertent overdose • tend to involve multiple agent ingestions General Concepts for Pediatric Poisoning • Prevention • Initial Stabilization • Diagnosis • Specific Antidotes Management of the Poisoned Child • Treat the Patient, Not the Poison --patient-specific treatment is safer, less expensive, and more effective Management of the Poisoned Child • Stabilization --Airway --Breathing --Circulation --Disability (neurologic) Management of the Poisoned Child • Respiratory Failure --airway obstruction from secretions, refluxed gastric contents, airway muscle relaxation --respiratory muscle rigidity --loss of respiratory drive --pulmonary edema Management of the Poisoned Child • Cardiovascular Collapse --arteriolar dilation --venous dilation --myocardial depression --dysrhythmias Management of the Poisoned Child • Neurologic -
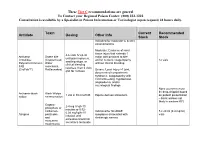
Antidote Stocking Tier C
These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Toxin Current Recommended Antidote Dosing Other Info Stock Stock Indicated for moderate to severe envenomations Moderate: Evidence of local tissue injury that extends 1 4-6 vials IV q2-4h Antivenin Snake bite major joint proximal to bite until pain improves, Crotalidae (Copperhead, and/or numeric coagulopathy 12 vials swelling stops, or Polyvalent Immune Water without clinical bleeding clinical bleeding FAB moccasins, resolves, then 2 vials (CroFab™) Rattlesnakes) Severe: Local injury >1 joint, q6h for 3 doses documented compartment syndrome, coagulopathy with clinical bleeding, hypotension, angioedema, and/or neurological findings None (currently must be drop-shipped based Antivenin-black Black Widow 1 vial in 50 ml of NS Equine derived antivenom on patient presentation widow envenomation – black widows not likely in western KY) Organo- 2-4 mg IV q5-10 phosphate or minutes or 0.02- carbamate Indicated for SLUDGE 5 x 20 ml (0.4 mg/ml) 0.08 mg/kg/hr IV Atropine pesticides symptoms associated with vials infusion until and cholinergic excess excessive bronchial muscarine secretions terminate mushrooms These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Antidote Current Recommended Toxin -

Antidotes Used in Poisonings and Ingestions Tip Sheet
UNC MEDICAL CENTER GUIDE Antidotes Used in Poisonings and Ingestions The purpose of this guide is to describe the locations and quantities of antidotes to be maintained at UNC Medical Center for poisonings and ingestions. Please refer to PHARM 0207: Antidote Availability for additional information. Chapel Hill Campus Hillsborough Campus Brand Generic Name Uses Name Location(s) Par Levels a Location(s) Par Levels Acetylcysteine (oral) Min: 18 Min: 18 Mucomyst Acetaminophen Talyst (IP) Talyst 30 mL Max: 36 Max: 36 Min: 22 Talyst Min: 22 Acetylcysteine (IV) Acetadote Acetaminophen Talyst (IP) Max: 33 Max: 33 Bites of crotalides Antivenin Crotalidae (rattlesnakes, Min: 20 Min: 20 CroFab Talyst (Refrig) Pass-through polyvalent (ovine) copperhead, Max: 24 Max: 24 cottonmouth moccasins) Antivenin Latrodectus mactans Black widow Min: 2 N/A Talyst (Refrig) Min: 2 b Talyst (Refrig) (Black Widow) spider bites Max: 2 (equine) Antivenin Micruus Call Regional Poison Call Regional Poison Fulvius (Coral N/A Coral snake bites c N/A c N/A Control Center Control Center Snake) Min: 29 Atropine 0.4 mg/mL; Cholinergic Min: 66 Talyst N/A Talyst (IP) Max: 44 1 mL vial agents Max: 99 Min: 13 Atropine 0.1mg/mL; Cholinergic Min: 22 Talyst; ED; Trauma Various Talyst (IP) Max: 23 10 mL syringe agents Max: 29 Botulism antitoxin Call CDC in Atlanta, Call CDC in Atlanta, N/A Botulism N/A N/A (ABE) GA d GA d Developed by: UNC Drug Information Center Page 1 of 5 Reviewed by: Pharmacy Operations Council, Sept. 2017 Updated: May 2018 Chapel Hill Campus Hillsborough Campus -

U.S. EPA, Pesticide Product Label, PACE TERGICIDE G GERMICIDE
An unusually efficient cleaner-even when 7930·985·6896 ACTIVE INGREDIENTS: Methyldodecylbenzyl used with hard water. Combined with a wide Trimethyl Ammonium Chloride ••••••••••••• 12. O~' spectrum disinfectant system, both systems Methyldodecylxylylene Bis (1'rimethyl Ammonium Chloride). • • • • • • • • • • • • . • • • • • . •• 3.070 being compaUble with each other to prevent Isopropanol •••.••••••.. " . .. • . • • • • • . .. 5 .0'10 loss of efficiency. TERGICIDE G Trisodium Phosphate...................... 0.5'/ T.etrasodium Ethylene Diamine Tetraacetate •• 0 .5 Z TERGICIDE, properly used, controls mixed hospital strains of pathogenic bacteria INERT INGREDIENTS ...................... 79.0'1 through: (1) bactericidal actiOn on many Ger.icWe .Id Deterl,.t bacteria: and (2) bacteriostatic action on many of the remaining bacteria by means of u .S. D.A. Reg. No. 4389-40 Phenol Coefficient by AOAC Method: a residual fUm deposited on treated surfaces. Staphylococcus aureus 45 P-D-00235 "-_~S~a:.::lll!0nella typhosa 35 Net Contents One Gal. RECOMtviEND£D PROCEDURES AMT. TERGICIDE GERMICIDE DILUTION FLOORS AND WALLS; To clean and sanit1ze-- RECOMMENDED 1'0 USE WITH CONCEN- USES EACH GAL. WATER TABLE TRATION 1. Remove loose soil by dry-mop or vacuum. Light soU deposits 1/2 oz. 1 : 250 600 ppm General disinfecting Manufactured by 2. Apply solution l1berall y and scrub with mop or machine. (The longer solution remains on sur PACIFIC CHEMICAL face. be tter the 'kill'.) For odor control , in public rooms 1/2 oz. I : 250 600 ppm and garbage areas Division of Pace Chemical Corp. 3. Pick-up soiled soluti0!1. 4. Rinse with clear wat~r if soil condition \';<.lrrants. HeavU y soUed areas Seattle, Washington HeavU y contaminated 1 oz. -

Preparation of Hydroxy Analogues of Several Amino Acids
PRE PARA TION OF HYDROXY ANA LOGUES OF SE\~RA L AMI NO ACIDS by THOMAS LE E ABRAHAMS A THESIS submitted to OREGON STA TE COLLEGE in partial fulfillment of the requirements for the degree of MAS TER OF SCIE NCE June 1957 APPROIIED t Redacted for Privacy Profcasor of Chcmlstry In Chsrgc of, !{aJsr lnd Heed of Chculatry Redacted for Privacy Chalrnen of $choo1 Oraduate 0onn!,ttor Redacted for Privacy Dren of 0mduata Sohool Date thoar.c ta prcso"t"a 7, / t y,fl,,Zf^*? fyptd by Barbsra Jr l{ol,coa ACK NOWLEDGMENT The author wishes to express his gratitude to Dr. Bert E. Christensen for his guidance and encouragement throughout the course of this research. TABLE OF CONTENTS Introduction .,.., -- ....... ... ... .. 1 Experimental .... - ... - - - 14 Summary~ __ ~ ~ ~ ~ ~ __ ........... - 21 Bibliography _____ - - .. - .. .. - 23 PREPARATION OF HYDROXY ANALOGUES OF SEVERAL AMINO ACIDS In 1911, Van Slyke (28, pp .l92-202} reported a procedure for the analysis of proteins by the determina tion of amino groups which are characteristic of most amino acids. Essentially , the method was adapted from the earlier Hausmann method (13, pp~l3S-145) as modified by Osborne and Harris (17, pp.,332-353) with his own meth od for the determination of amino nitrogen. In his orig inal study dealing with the method, Van Slyke (28, pp .l92 202) showed that the majority of the naturally occurring amino acids with the exception of glycine and cystine~ when treated with nitrous acid, yield their amino nitro gen quantitatively at room temperature. More recently,