Hazardous Drug Acknowledgement Statement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Specialty Drug List 10-22-12 Final
Iowa Medicaid Specialty Drug List Effective 10/22/2012 “Specialty” drugs include biological drugs, blood-derived products, complex molecules, and select oral, injectable, and infused medications identified by the Department and reimbursed at AWP-17% plus the dispensing fee. Specialty pricing will be applied to both the brand and generic drug products. NOTE: See the PDL at www.iowamedicaidpdl.com for specific PA criteria for the following drugs. BRAND NAME GENERIC NAME AGENTS FOR GAUCHER DISEASE ELELYSO taliglucerase alfa VPRIV velaglucerase alfa ALS RILUTEK riluzole ALCOHOL DEPENDENCE VIVITROL naltrexone AMINOGLYCOSIDES TOBI tobramycin ANTI-ASTHMATICS ALPHA PROTEINASE INHIBITORS ARALAST proteinase inhibitor ARALAST NP proteinase inhibitor PROLASTIN, C proteinase inhibitor ZEMAIRA alpha-1 proteinase inhibitor ANTIASTHMATIC - BETA - ADRENERGICS BRETHINE INJECTION turbutaline sulfate ANTIASTHMATIC - HYDRO-LYTIC ENZYMES KALYDECO ivacaftor ANTIBIOTICS - MISC. CAYSTON aztreonam lysine for inhal soln ANTI-CATAPLECTIC AGENTS XYREM sodium oxybate oral solution ANTICOAGULANTS ARIXTRA fondaparinux sodium FRAGMIN dalteparin sodium INNOHEP tinzaparin sodium LOVENOX enoxaparin sodium ANTICONVULSANTS SABRIL vigabatrin ANTIDOTES FERRIPROX deferiprone ANTIDOTES - CHELATING AGENTS CHEMET succimer EXJADE deferasirox tab for oral susp Page 1 of 10 ANTIEMETIC - TETRAHYDROCANNABINOL (THC) DERIVATIVES MARINOL dronabinol ANTIFUNGALS - ASSORTED AMBISOME amphotericin B Liposome IV for suspension ANCOBON flucytosine CANCIDAS caspofungin acetate for IV solution -

Ocps) Used in the NM Family Planning Program (FPP
The following slides are intended to familiarize nurses and clinicians with oral contraceptive pills (OCPs) used in the NM Family Planning Program (FPP). The FPP does not intend for this information to supersede the Family Planning Protocol particularly on the requirement for Public Health Nurses in the Public Health Offices to consult a clinician as stated in the Protocol when in doubt or if it is necessary to switch the client’s OCP type. 1 2 Combined oral contraceptives (COCs) contain two hormones; estrogen and progestin. In general, any combined OCP is good for most women who are eligible to take estrogen according to the CDC U.S. Medical Eligibility Criteria (MEC). Once again, refer to the US MEC chart to find out if OCP is a suitable choice for clients with specific health conditions. To learn a little bit about what each hormone does, the FPP is providing the following summary: Estrogen: provides endometrial stability = menstrual cycle control. A higher estrogen dose increases the venous thromboembolism (VTE) or clot risk but OCP clot risk is still less harmful than the clot risk related to pregnancy and giving birth. Progestin: provides most of the contraceptive effect by ‐Preventing luteinizing hormone (LH) surge /ovulation ‐Thickening the cervical mucus to prevent sperm entry. Two major OCP formations are available. Monophasic: There is only one dose of estrogen and progestin in each active pill in the packet; and Multiphasic: There are varying doses of hormones, particularly progestin in the active pills. 3 Section 3 of the FPP Protocol contains the OCP Substitute Table, which groups OCPs into 6 classes according to the estrogen dosage, the type of progestin and the formulations. -

Aromasin (Exemestane)
HIGHLIGHTS OF PRESCRIBING INFORMATION ------------------------------ADVERSE REACTIONS------------------------------ These highlights do not include all the information needed to use • Early breast cancer: Adverse reactions occurring in ≥10% of patients in AROMASIN safely and effectively. See full prescribing information for any treatment group (AROMASIN vs. tamoxifen) were hot flushes AROMASIN. (21.2% vs. 19.9%), fatigue (16.1% vs. 14.7%), arthralgia (14.6% vs. 8.6%), headache (13.1% vs. 10.8%), insomnia (12.4% vs. 8.9%), and AROMASIN® (exemestane) tablets, for oral use increased sweating (11.8% vs. 10.4%). Discontinuation rates due to AEs Initial U.S. Approval: 1999 were similar between AROMASIN and tamoxifen (6.3% vs. 5.1%). Incidences of cardiac ischemic events (myocardial infarction, angina, ----------------------------INDICATIONS AND USAGE--------------------------- and myocardial ischemia) were AROMASIN 1.6%, tamoxifen 0.6%. AROMASIN is an aromatase inhibitor indicated for: Incidence of cardiac failure: AROMASIN 0.4%, tamoxifen 0.3% (6, • adjuvant treatment of postmenopausal women with estrogen-receptor 6.1). positive early breast cancer who have received two to three years of • Advanced breast cancer: Most common adverse reactions were mild to tamoxifen and are switched to AROMASIN for completion of a total of moderate and included hot flushes (13% vs. 5%), nausea (9% vs. 5%), five consecutive years of adjuvant hormonal therapy (14.1). fatigue (8% vs. 10%), increased sweating (4% vs. 8%), and increased • treatment of advanced breast cancer in postmenopausal women whose appetite (3% vs. 6%) for AROMASIN and megestrol acetate, disease has progressed following tamoxifen therapy (14.2). respectively (6, 6.1). ----------------------DOSAGE AND ADMINISTRATION----------------------- To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc at Recommended Dose: One 25 mg tablet once daily after a meal (2.1). -

Assessment of Overall Survival, Quality of Life, And
Supplementary Online Content Salas-Vega S, Iliopoulos O, Mossialos E. Assesment of overall survival, quality of life, and safety benefits associated with new cancer medicines [published online December 29, 2016]. JAMA Oncol. doi:10.1001/jamaoncol.2016.4166 eTable 1. Therapeutic profile of all cancer medicines approved by the FDA between 2003- 2013 eTable 2. Regulatory evidence in support of classification of drug clinical benefits eFigure 1. Number of cancer drugs that were evaluated by all three HTA agencies, sorted by magnitude of clinical benefits eTable 3. Interagency agreement–Krippendorff’s alpha coefficients eMethods This supplementary material has been provided by the authors to give readers additional information about their work. Online-Only Supplement © 2016 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/25/2021 Clinical value of cancer medicines Contents eExhibits ......................................................................................................................................................... 3 eTable 1. Therapeutic profile of all cancer medicines approved by the FDA between 2003- 2013 (Summary of eTable 2) ................................................................................................................... 3 eTable 2. Regulatory evidence in support of classification of drug clinical benefits ....................... 6 eFigure 1. Number of cancer drugs that were evaluated by all three HTA agencies, sorted by magnitude of clinical benefits -

The Chemotherapy of Malignant Disease -Practical and Experimental Considerations
Postgrad Med J: first published as 10.1136/pgmj.41.475.268 on 1 May 1965. Downloaded from POSTGRAD. MED. J. (1965), 41,268 THE CHEMOTHERAPY OF MALIGNANT DISEASE -PRACTICAL AND EXPERIMENTAL CONSIDERATIONS JOHN MATTHIAS, M.D., M.R.C.P., F.F.A., R.C.S. Physician, The Royal Marsden Hospital, London, S.W.3. THE TERM chemotherapy was introduced by positively charged alkyl (CH2) radicles of Ehrlich to describe the specific and effective the agent. treatment of infectious disease by chemical (a) The nitrogen mustards: mustine (HN2 substances. It is currently also applied to the 'nitrogen mustard', mechlorethamine, treatment of malignant disease. Unfortunately mustargen), trimustine (Trillekamin no aspect of tumour metabolism has been HN3), chlorambucil (Leukeran, phenyl discovered which has allowed the development butyric mustard), melphalan (Alkeran, of drugs capable of acting specifically upon the phenyl alanine mustard), uramustine malignant cell, so that cytotoxic drugs also (Uracil mustard), cyclophosphamide affect normal cells to a greater or lesser degree. (Endoxan or Cytoxan), mannomustine The most susceptible or sensitive of the normal (DegranoO). tissues are those with the highest rates of cell (b) The ethylenamines: tretamine (trie- turnover and include the haemopoietic and thanomelamine, triethylene melamine, lympho-reticular tissues, the gastro-intestinal TEM), thiotepa (triethylene thiopho- the the testis and the hair epithelium, ovary, sphoramide), triaziquone (Trenimon).by copyright. follicles. (c) The epoxides: triethyleneglycoldigly- Cancer chemotherapy may be said to encom- cidyl ether (Epodyl). pass all treatments of a chemical nature (d) The sulphonic acid esters: busulphan administered to patients with the purpose of (Myleran), mannitol myleran. restricting tumour growth or destroying tumour 2. -

Comparing the Effects of Combined Oral Contraceptives Containing Progestins with Low Androgenic and Antiandrogenic Activities on the Hypothalamic-Pituitary-Gonadal Axis In
JMIR RESEARCH PROTOCOLS Amiri et al Review Comparing the Effects of Combined Oral Contraceptives Containing Progestins With Low Androgenic and Antiandrogenic Activities on the Hypothalamic-Pituitary-Gonadal Axis in Patients With Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis Mina Amiri1,2, PhD, Postdoc; Fahimeh Ramezani Tehrani2, MD; Fatemeh Nahidi3, PhD; Ali Kabir4, MD, MPH, PhD; Fereidoun Azizi5, MD 1Students Research Committee, School of Nursing and Midwifery, Department of Midwifery and Reproductive Health, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran 2Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran 3School of Nursing and Midwifery, Department of Midwifery and Reproductive Health, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran 4Minimally Invasive Surgery Research Center, Iran University of Medical Sciences, Tehran, Islamic Republic Of Iran 5Endocrine Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran Corresponding Author: Fahimeh Ramezani Tehrani, MD Reproductive Endocrinology Research Center Research Institute for Endocrine Sciences Shahid Beheshti University of Medical Sciences 24 Parvaneh Yaman Street, Velenjak, PO Box 19395-4763 Tehran, 1985717413 Islamic Republic Of Iran Phone: 98 21 22432500 Email: [email protected] Abstract Background: Different products of combined oral contraceptives (COCs) can improve clinical and biochemical findings in patients with polycystic ovary syndrome (PCOS) through suppression of the hypothalamic-pituitary-gonadal (HPG) axis. Objective: This systematic review and meta-analysis aimed to compare the effects of COCs containing progestins with low androgenic and antiandrogenic activities on the HPG axis in patients with PCOS. -

PMBJP Product.Pdf
Sr. Drug Generic Name of the Medicine Unit Size MRP Therapeutic Category No. Code Analgesic & Antipyretic / Muscle 1 1 Aceclofenac 100mg and Paracetamol 325 mg Tablet 10's 10's 8.00 relaxants Analgesic & Antipyretic / Muscle 2 2 Aceclofenac Tablets IP 100mg 10's 10's 4.37 relaxants Acetaminophen 325 + Tramadol Hydrochloride 37.5 film Analgesic & Antipyretic / Muscle 3 4 10's 8.00 coated Tablet 10's relaxants Analgesic & Antipyretic / Muscle 4 5 ASPIRIN Tablets IP 150 mg 14's 14's 2.70 relaxants DICLOFENAC 50 mg+ PARACETAMOL 325 mg+ Analgesic & Antipyretic / Muscle 5 6 10's 11.30 CHLORZOXAZONE 500 mg Tablets 10's relaxants Diclofenac Sodium 50mg + Serratiopeptidase 10mg Tablet Analgesic & Antipyretic / Muscle 6 8 10's 12.00 10's relaxants Analgesic & Antipyretic / Muscle 7 9 Diclofenac Sodium (SR) 100 mg Tablet 10's 10's 6.12 relaxants Analgesic & Antipyretic / Muscle 8 10 Diclofenac Sodium 25mg per ml Inj. IP 3 ml 3 ml 2.00 relaxants Analgesic & Antipyretic / Muscle 9 11 Diclofenac Sodium 50 mg Tablet 10's 10's 2.90 relaxants Analgesic & Antipyretic / Muscle 10 12 Etoricoxilb Tablets IP 120mg 10's 10's 33.00 relaxants Analgesic & Antipyretic / Muscle 11 13 Etoricoxilb Tablets IP 90mg 10's 10's 25.00 relaxants Analgesic & Antipyretic / Muscle 12 14 Ibuprofen 400 mg + Paracetamol 325 mg Tablet 10's 15's 5.50 relaxants Analgesic & Antipyretic / Muscle 13 15 Ibuprofen 200 mg film coated Tablet 10's 10's 1.80 relaxants Analgesic & Antipyretic / Muscle 14 16 Ibuprofen 400 mg film coated Tablet 10's 15's 3.50 relaxants Analgesic & Antipyretic -

ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (Norgestimate/Ethinyl Estradiol)
PHYSICIANS' PACKAGE INSERT ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (norgestimate/ethinyl estradiol) Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. DESCRIPTION Each of the following products is a combination oral contraceptive containing the progestational compound norgestimate and the estrogenic compound ethinyl estradiol. ORTHO TRI-CYCLEN 21 Tablets and ORTHO TRI-CYCLEN 28 Tablets. Each white tablet contains 0.180 mg of the progestational compound, norgestimate (18,19-Dinor- 17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-, oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include lactose, magnesium stearate, and pregelatinized starch. Each light blue tablet contains 0.215 mg of the progestational compound norgestimate (18,19- Dinor-17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. Each blue tablet contains 0.250 mg of the progestational compound norgestimate (18,19-Dinor-17- pregn-4-en-20-yn-3-one, 17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. -

Palifermin (Kepivance™) in the Treatment of Mucositis
Session V • From cell biology to cell therapy Palifermin (Kepivance™) in the treatment of mucositis [haematologica reports] 2005;1(8):41-45 EMMANOUILIDES C n patients undergoing high dose chemo- factor (KGF) is a 28 kD member of the therapy and hematopoietic stem cell fibroblast growth factor family with epithe- Associate Profesor transplant, oral mucositis (OM) is one of lial cell proliferative properties.26 Palifermin of Medicine, UCLA, I Division Haematology-Oncology, the most debilitating and annoying side (Kepivance™) is a truncated, recombinant Thessaloniki, Greece effects. This complication results from form of human keratinocyte growth factor cytotoxic injury to the epithelial lining of (rHuKGF) that has been approved in the the oropharyngeal mucosa, although USA in 2004 to decrease the incidence and lesions of the whole gastrointestinal tract duration of severe OM in patients with also occur.1 The severity of OM varies from hematologic malignancies receiving myelo- erythema and edema accompanied by mild toxic therapy requiring HSCT support. Pal- soreness to full mucosal thickness ulcera- ifermin (recombinant human keratinocyte tions penetrating into the submucosa, growth factor) is an N-terminal, truncated often resulting in severe pain requiring version of endogenous keratinocyte growth narcotic analgesia and impaired swallow- factor with biologic activity similar to that ing, prolonged hospitalization, and of the native protein, but with increased increased risks for infections and poten- stability 26 In animal models of chemother- tially life-threatening sequelae.2,3 Between apy, radiotherapy, and hematopoietic stem- 40% to 80% of cancer patients undergo- cell transplantation27,28 palifermin protected ing intensive treatment regimens requiring several types of epithelial tissues. -
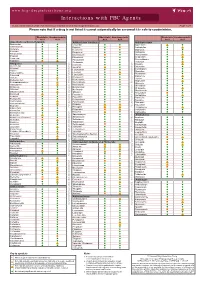
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created March 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Fluvoxamine Lymecycline distribution. Celecoxib Imipramine Meropenem Codeine Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin only. Not for distribution. for only. Not Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Trazodone Ibuprofen Rifampicin -

4 Supplementary File
Supplemental Material for High-throughput screening discovers anti-fibrotic properties of Haloperidol by hindering myofibroblast activation Michael Rehman1, Simone Vodret1, Luca Braga2, Corrado Guarnaccia3, Fulvio Celsi4, Giulia Rossetti5, Valentina Martinelli2, Tiziana Battini1, Carlin Long2, Kristina Vukusic1, Tea Kocijan1, Chiara Collesi2,6, Nadja Ring1, Natasa Skoko3, Mauro Giacca2,6, Giannino Del Sal7,8, Marco Confalonieri6, Marcello Raspa9, Alessandro Marcello10, Michael P. Myers11, Sergio Crovella3, Paolo Carloni5, Serena Zacchigna1,6 1Cardiovascular Biology, 2Molecular Medicine, 3Biotechnology Development, 10Molecular Virology, and 11Protein Networks Laboratories, International Centre for Genetic Engineering and Biotechnology (ICGEB), Padriciano, 34149, Trieste, Italy 4Institute for Maternal and Child Health, IRCCS "Burlo Garofolo", Trieste, Italy 5Computational Biomedicine Section, Institute of Advanced Simulation IAS-5 and Institute of Neuroscience and Medicine INM-9, Forschungszentrum Jülich GmbH, 52425, Jülich, Germany 6Department of Medical, Surgical and Health Sciences, University of Trieste, 34149 Trieste, Italy 7National Laboratory CIB, Area Science Park Padriciano, Trieste, 34149, Italy 8Department of Life Sciences, University of Trieste, Trieste, 34127, Italy 9Consiglio Nazionale delle Ricerche (IBCN), CNR-Campus International Development (EMMA- INFRAFRONTIER-IMPC), Rome, Italy This PDF file includes: Supplementary Methods Supplementary References Supplementary Figures with legends 1 – 18 Supplementary Tables with legends 1 – 5 Supplementary Movie legends 1, 2 Supplementary Methods Cell culture Primary murine fibroblasts were isolated from skin, lung, kidney and hearts of adult CD1, C57BL/6 or aSMA-RFP/COLL-EGFP mice (1) by mechanical and enzymatic tissue digestion. Briefly, tissue was chopped in small chunks that were digested using a mixture of enzymes (Miltenyi Biotec, 130- 098-305) for 1 hour at 37°C with mechanical dissociation followed by filtration through a 70 µm cell strainer and centrifugation. -

Estrogen and Progestin Hormone Doses in Combined Birth Control Pills
Estrogen and Progestin Hormone Doses in Combined Birth Control Pills Estrogen level Pill Brand Name Progestin Dose (mg) ethinyl estradiol (micrograms) 20 mcgm Alesse® levonorgestrel 0.10 Levlite® levonorgestrel 0.10 Loestrin 1/20® Fe norethindrone 1.00 acetate Mircette® desogestrel 0.15 Ortho Evra® norelgestromin 0.15 (patch) (norgestimate metabolite) phasic Estrostep® Fe norethindrone 1.0/1.0/1.0 20/30/35 mcgm acetate 30 mcgm Levlen® levonorgestrel 0.15 Levora® levonorgestrel 0.15 Nordette® levonorgestrel 0.15 Lo/Ovral® norgestrel 0.30 Desogen® desogestrel 0.15 Ortho-Cept® desogestrel 0.15 Loestrin® 1.5/30 norethindrone 1.50 acetate Yasmin® drospirenone 3.0 phasic Triphasil® levonorgestrel 0.05/0.075/0.125 30/40/30 mcgm Tri-Levlen® levonorgestrel 0.05/0.075/0.125 Trivora® levonorgestrel 0.05/0.075/0.125 35 mcgm Ortho-Cyclen® norgestimate 0.25 Ovcon-35® norethindrone 0.40 Brevicon® norethindrone 0.50 Modicon® norethindrone 0.50 Necon® norethindrone 1.00 Norethin® norethindrone 1.00 Norinyl® 1/35 norethindrone 1.00 Ortho-Novum® 1/35 norethindrone 1.00 Demulen® 1/35 ethynodiol diacetate 1.00 Zovia® 1/35E ethynodiol diacetate 1.00 phasic Ortho-Novum® norethindrone 0.50/1.00 35/35 mcgm 10/11 Jenest® norethindrone 0.50/1.00 phasic Ortho-Tri-Cyclen® norgestimate 0.15/0.215/0.25 35/35/35 mcgm Ortho-Novum® norethindrone 0.50/0.75/1.00 7/7/7 Tri-Norinyl® norethindrone 0.50/1.00/0.50 50 mcgm Necon® 1/50 norethindrone 1.00 Norinyl® 1/50 norethindrone 1.00 Ortho-Novum® 1/50 norethindrone 1.00 Ovcon-50® norethindrone 1.00 Ovral® norgestrel 0.50 Demulen® 1/50 ethynodiol diacetate 1.00 Zovia® 1/50E ethynodiol diacetate 1.00 Which pills have higher progestin side efects or cause more acne and hair growth? Each progestin has a diferent potency, milligram per milligram, in terms of progesterone efect to stop menstrual bleeding or androgen efect to stimulate acne and hair growth.