Quilicura Report Final
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Arauco Quilicura Becomes the First Chilean Mall to Obtain LEED Certification
Arauco Quilicura becomes the first Chilean mall to obtain LEED certification Santiago, February 20, 2015 - The Green Building Certification Institute (GBCI) has awarded the LEED (Leadership in Energy and Environmental Design) certification, Silver category, to Arauco Quilicura. This is an important milestone for Parque Arauco as this is the first shopping center in Chile and the first open-air shopping center in Latin America to receive this recognition. “During our more than three decades of history, innovation and cutting-edge design have been present in our projects,” said Andrés Torrealba, CEO of Parque Arauco´s Chile Division. “The LEED certification is proof of this, and we are very proud of this recognition, especially given that we did not set out to receive the certification, but were only using the same standards we have in place for all our projects while using LEED as a reference to improve the design. This process enabled us to become the first shopping center in Chile to earn this certification.” Sarah Alexander, Vice President of Certification for GBCI, made special mention of the LEED Silver certification obtained by Parque Arauco: “Mall Arauco Quilicura is a showcase example of sustainable design and demonstrates its leadership in transforming the building industry. Mall Arauco Quilicura is the first shopping center to earn a LEED certification in Chile. This is a tremendous accomplishment.” Arauco Quilicura was developed as an environmentally friendly project in terms of design, construction and operation. The sustainable practices implemented by the shopping mall will enable a reduction of water consumption of up to 29.9%, a reduction of energy consumption of up to 13%, and a treatment plan for non-organic waste. -

Primer Tribunal Electoral De La Region Metropolitana
PRIMER TRIBUNAL ELECTORAL DE LA REGIÓN METROPOLITANA ESTADO CONFORME AL ART. 27 LEY N° 18.593 Pág.1/3 Santiago, 11 de octubre de 2018 ORDEN ROL N° ROL (en letras) RECLAMANTE - MATERIA RESOLUCIONES 1 6224 Seis mil doscientos veinticuatro Junta de Vecinos “Amapolas” de la comuna de Ñuñoa. Calificación elecciones. Sentencia 2 6321 Seis mil trescientos veintiuno CDL de Salud “Cesfam Quinta Bella” de la comuna de Recoleta. Calificación elecciones. Sentencia 3 6375 Seis mil trescientos setenta y cinco Club Deportivo “Fernando Collinao” de la comuna de Lo Prado. Calificación elecciones. Sentencia 4 6458 Seis mil cuatrocientos cincuenta y ocho Club de Adulto Mayor “Comienzo de una Vida” de la comuna de La Cisterna. Calificación elecciones. Sentencia Club Deportivo y Social “Seniors Villa España” de la comuna de Estación Central. Calificación 5 6463 Seis mil cuatrocientos sesenta y tres elecciones Sentencia 6 6465 Seis mil cuatrocientos sesenta y cinco Comité de Allegados “Juntos por Nuestra Casa” de la comuna de La Granja. Calificación elecciones. Sentencia Club Adulto Mayor, Cultural y Social “Los Años Dorados” de la comuna de Huechuraba. Calificación 7 6466 Seis mil cuatrocientos sesenta y seis elecciones. Sentencia 8 6468 Seis mil cuatrocientos sesenta y ocho Centro Cultural “Comunicacional TV 8 Peñalolén” de la comuna de Peñalolén. Calificación elecciones. Sentencia 9 6473 Seis mil cuatrocientos setenta y tres Club del Adulto Mayor “Pila del Ganso” de la comuna de Estación Central. Calificación elecciones. Sentencia Centro de Desarrollo Social “Esperanza del Mañana” de la comuna de La Granja. Calificación 10 6503 Seis mil quinientos tres elecciones. Sentencia 11 6607 Seis mil seiscientos siete Comité de Allegados “Fuerza y Coraje” de la comuna de Puente Alto. -

Quinta Normal Matucana Pudahuel Quinta Normal Gruta De Lourdes Av
Learn about the routes nearby this station 109 406 422 426 505 507 508 513 Maipú Cantagallo La Reina La Dehesa Las Parcelas Grecia Las Torres José Arrieta Quinta Normal Matucana Pudahuel Quinta Normal Gruta de Lourdes Av. Mapocho Gruta de Lourdes Gruta de Lourdes Estación Central Quinta Normal Sn. Pablo-Matucana Est. Central-Alameda Compañía-Pza. Italia Est. Central-Av. España Compañía-Pza. Italia Compañía-Pza. Italia Cam. a Melipilla Est. Central-Alameda Alameda-Portugal Av. Providencia Av. Salvador Pque. O'Higgins Av. Salvador-Av. Grecia Av. Salvador 1 5 de Abril Av. Providencia Av. Irarrázaval Av. Las Condes Av. Irarrázaval Av. Matta-Av. Grecia Jorge Monckeberg Av. Irarrázaval Pza. de Maipú Av. Las Condes Hospital Militar Cantagallo Av. Las Parcelas Estadio Nacional Las Torres Plaza Egaña Villa San Luis Cantagallo Villa La Reina La Dehesa Río Claro Muni. Peñalolén Av. Departamental J. Arrieta-Río Claro 6 PA1 Quinta Normal J16 B26 B28 J01 J02 J05 Estación Central Estación Central Quinta Normal Quinta Normal U.L.A. U.L.A. Costanera Sur Panamericana Plaza Renca San Pablo San Francisco Salvador Gutiérrez Hosp. Félix Bulnes Puente Bulnes Hosp. Félix Bulnes Neptuno-Av. Carrascal Neptuno Gruta de Lourdes Radal Matucana Av. Mapocho Hosp. Félix Bulnes José Joaquín Pérez Av. San Pablo Gruta de Lourdes Quinta Normal Matucana Av. San Pablo Quinta Normal Matucana Quinta Normal Hosp. San Juan de Dios Quinta Normal Quinta Normal Esperanza Quinta Normal Estación Central Estación Central Hosp. San Juan de Dios Hosp. San Juan de Dios U.L.A. U.L.A. 2 4 expreso expreso J01 J02 J05 J16 313e 313e Pudahuel Sur Enea Costanera Sur Costanera Sur Estación Central Quilicura Santo Domingo Quinta Normal Maipú Matucana San Ignacio Gral. -
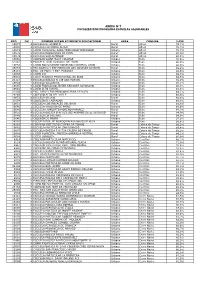
Rbd Dv Nombre Establecimiento
ANEXO N°7 FOCALIZACIÓN PROGRAMA ESCUELAS SALUDABLES RBD DV NOMBRE ESTABLECIMIENTO EDUCACIONAL AREA COMUNA %IVE 10877 4 ESCUELA EL ASIENTO Rural Alhué 74,7% 10880 4 ESCUELA HACIENDA ALHUE Rural Alhué 78,3% 10873 1 LICEO MUNICIPAL SARA TRONCOSO TRONCOSO Urbano Alhué 78,7% 10878 2 ESCUELA BARRANCAS DE PICHI Rural Alhué 80,0% 10879 0 ESCUELA SAN ALFONSO Rural Alhué 90,3% 10662 3 COLEGIO SAINT MARY COLLEGE Urbano Buin 76,5% 31081 6 ESCUELA SAN IGNACIO DE BUIN Urbano Buin 86,0% 10658 5 LICEO POLIVALENTE MODERNO CARDENAL CARO Urbano Buin 86,0% 26015 0 ESC.BASICA Y ESP.MARIA DE LOS ANGELES DE BUIN Rural Buin 88,2% 26111 4 ESC. DE PARV. Y ESP. PUKARAY Urbano Buin 88,6% 10638 0 LICEO 131 Urbano Buin 89,3% 25591 2 LICEO TECNICO PROFESIONAL DE BUIN Urbano Buin 89,5% 26117 3 ESCUELA BÁSICA N 149 SAN MARCEL Urbano Buin 89,9% 10643 7 ESCUELA VILLASECA Urbano Buin 90,1% 10645 3 LICEO FRANCISCO JAVIER KRUGGER ALVARADO Urbano Buin 90,8% 10641 0 LICEO ALTO JAHUEL Urbano Buin 91,8% 31036 0 ESC. PARV.Y ESP MUNDOPALABRA DE BUIN Urbano Buin 92,1% 26269 2 COLEGIO ALTO DEL VALLE Urbano Buin 92,5% 10652 6 ESCUELA VILUCO Rural Buin 92,6% 31054 9 COLEGIO EL LABRADOR Urbano Buin 93,6% 10651 8 ESCUELA LOS ROSALES DEL BAJO Rural Buin 93,8% 10646 1 ESCUELA VALDIVIA DE PAINE Urbano Buin 93,9% 10649 6 ESCUELA HUMBERTO MORENO RAMIREZ Rural Buin 94,3% 10656 9 ESCUELA BASICA G-N°813 LOS AROMOS DE EL RECURSO Rural Buin 94,9% 10648 8 ESCUELA LO SALINAS Rural Buin 94,9% 10640 2 COLEGIO DE MAIPO Urbano Buin 97,9% 26202 1 ESCUELA ESP. -

Ccr-Centro-Cultural-Renca.Pdf (3.992Mb)
MEMORIA DE TITULO Estudiante:Camila Medina Alarcón - Profesor Guía: Rodrigo Chauriye CCRCENTRO CULTURAL RENCA REGION METROPOLITANA-COMUNA DE RENCA GRACIASTOTALES INDICE CAPITULO I - INTRODUCCIÓN AL TEMA Intrucccion 09 Jus fi cacion y mo vaciones 11 Problema y Diagnos co 13 Obje vos 15 CAPITULO II- MARCO TEORICO Marco Teorico 19 Revitalizacion de los Cerros de San ago 21 Cerros Isla: Cerros de Renca 23 Situacion Cultural de Renca 25 Colec vos Culturales 26 CAPITULO III - EMPLAZAMIENTO Renca 35 Contexto Historico 41 Entorno Construido 43 CAPITULO IV - PROYECTO Insercion del proyecto 49 Escalas del proyecto 51 Modelo de Ges on y mantenimiento 54 Sustentabilidad 56 Propuesta Programa ca 58 Defi nicion del Usuario 60 Funcionalidad y Espacialidad 62 Planimetria 63 Referentes 67 Conclusiones 69 Bibliogra a 71 CAPITULO I INTRODUCCION AL TEMA INTRODUCCIÓN En el proceso como estudiante de Arquitectura llevo a que tuviese un especial interés en temas sociales y como estos infl uyen en la ciudad. En este sen do las estrategias de aproximación al proyecto pasa- ron, primero que todo, por un análisis general de la ciudad de San- ago, viendo las fortalezas y necesidades de cada comuna, siendo lo sufi cientemente interesante para este importante proceso. Es así como se llega a la comuna de Renca. Renca es una comuna que se encuentra en el noreste de la comu- na de San ago. Debido a la expansión urbana de la ciudad, esta ha ido quedando aislada tanto naturalmente como por la interven- ción del hombre. An guamente esta aislación, tanto por el Cerro Renca como por el río Mapocho, era favorable ya que se generaba un refugio natural de los indígenas del lugar, donde podían cul - var y protegerse de los españoles. -

Estimación Población Extranjera En Chile 2019 Regiones Y Comunas
Estimación de personas extranjeras residentes habituales en Chile al 31 de diciembre de 2019. Informe técnico: desagregación regional y comunal. Instituto Nacional de Estadísticas Departamento de Extranjería y Migración Junio/2020 CONTENIDO I. RESUMEN ................................................................................................................................. 3 II. INTRODUCCIÓN ...................................................................................................................... 4 III. ANTECEDENTES ................................................................................................................ 6 3.1 Objetivos ................................................................................................................................. 6 3.2 Marco conceptual ................................................................................................................... 6 3.3 Fuentes de información ........................................................................................................ 9 IV. Metodología de estimación: .............................................................................................. 10 V. Procesamiento del componente censal .............................................................................. 12 VI. Procesamiento del componente de registros administrativos ..................................... 14 VII. Principales resultados ....................................................................................................... -

Horario Y Mapa De La Línea 435 De Micro
Horario y mapa de la línea 435 de micro 435 Quilicura Ver En Modo Sitio Web La línea 435 de micro (Quilicura) tiene 2 rutas. Sus horas de operación los días laborables regulares son: (1) a Quilicura: 6:30 - 22:35 (2) a Vitacura: 5:30 - 22:22 Usa la aplicación Moovit para encontrar la parada de la línea 435 de micro más cercana y descubre cuándo llega la próxima línea 435 de micro Sentido: Quilicura Horario de la línea 435 de micro 34 paradas Quilicura Horario de ruta: VER HORARIO DE LA LÍNEA lunes 6:30 - 22:35 martes 6:30 - 22:35 Pc332-Av. Pdte. Kennedy / Esq. Av. Las Tranqueras miércoles 6:30 - 22:35 8028 Avenida Presidente Kennedy, Santiago jueves 6:30 - 22:35 Pc1121-Las Tranqueras / Esq. Virginia viernes 6:30 - 22:35 8201 Virginia, Santiago sábado 6:30 - 21:45 Pc376-Avenida Vitacura / Esq. Lo Gallo 8362 Avenida Vitacura, Santiago domingo 5:30 - 21:00 Pc251-Avenida Vitacura / Esq. Lo Gallo 8064 Avenida Vitacura, Santiago Pc252-Avenida Vitacura / Esq. Partenon Información de la línea 435 de micro 7564 Avenida Vitacura, Santiago Dirección: Quilicura Paradas: 34 Pc253-Avenida Vitacura / Esq. Carmen Fariña Duración del viaje: 50 min 7402 Avenida Vitacura, Santiago Resumen de la línea: Pc332-Av. Pdte. Kennedy / Esq. Av. Las Tranqueras, Pc1121-Las Tranqueras / Pc254-Avenida Vitacura / Esq. Miguel Comas Esq. Virginia, Pc376-Avenida Vitacura / Esq. Lo 1761 Miguel Comas, Santiago Gallo, Pc251-Avenida Vitacura / Esq. Lo Gallo, Pc252-Avenida Vitacura / Esq. Partenon, Pc253- Pc1082-Parada 2 / Los Cobres De Vitacura Avenida Vitacura / Esq. -

“Acciones De Participación Que Las Comunas De Recoleta, Quilicura Y Santiago Realizan Para La Inclusión De Los Migrantes”
FACULTAD DE CIENCIAS SOCIALES DEPARTAMENTO DE CIENCIA POLÍTICA Y RELACIONES INTERNACIONALES MAGISTER EN GOBIERNO, POLÍTICAS PÚBLICAS Y TERRITORIO “Acciones de Participación que Las Comunas de Recoleta, Quilicura y Santiago Realizan para la Inclusión de los Migrantes” Memoria para optar al grado de Magíster en Gobierno, Políticas Públicas y Territorio Estudiante Isabel Carvajal Gamonal Director de Trabajo de Graduación Natalia Hernandez Profesor Informante Fabian Pressacco Santiago, Chile, Abril 2018 INDICE 1. INTRODUCCIÓN 4 2. PROBLEMATIZACIÓN 7 Realidad migratoria en Chile 7 El rol de lo local en la inclusión de migrantes 9 Inclusión desde la iniciativa de la población y ciudadanía 12 3. DESARROLLO DEL TEMA 15 Migración bajo un enfoque de Derechos 15 Migración en Chile 17 Marco legal de la migración en Chile 19 Migración: Inclusión y exclusión 20 La multidimensionalidad de la inclusión. 22 Participación local de migrantes 29 2 4. OBJETIVOS 30 Objetivo General 30 Objetivos Específicos 30 5. PREGUNTA DE INVESTIGACIÓN 30 6. METODOLOGÍA 31 Fuentes de información 31 Dimensiones de interés 32 Instrumentos de recolección de información 34 Análisis de la información 34 7. ANÁLISIS DE LA INFORMACIÓN 35 Análisis 1 35 Análisis 2 48 8. CONCLUSIONES 53 9. CITAS BIBLIOGRPÁFICAS 57 10. ANEXOS 58 3 1. INTRODUCCIÓN En las últimas décadas, los índices de migración en Chile se han incrementado notoriamente. En efecto, Chile es el país de Sudamérica que incrementó mayormente su número de inmigrantes (84%), casi triplicándose entre 1990 y 2013. Actualmente, según cifras del Departamento de Extranjeria y Migración del Ministerio del Interior, existen 600.000 extranjeros residiendo en el país y de ellos 441.000 poseen la calidad de residentes regulares (Ministerio del Interior y Seguridad Pública, 2015). -

Sostenibilidad Urbana En La Comuna De Santiago De Chile
AE-OBSV. Observatorios de Sostenibilidad. Iniciativas Españolas e Iberoamericanas SOSTENIBILIDAD URBANA EN LA COMUNA DE SANTIAGO DE CHILE Néstor Ahumada Galaz Técnico Asesoría Urbana ASESORÍA URBANA MUNICIPALIDAD DE SANTIAGO DE CHILE www.conama9.org Sostenibilidad Urbana en la Comuna de Santiago de Chile Néstor Ahumada Galaz Geógrafo I. Municipalidad de Santiago Asesoría Urbana Santiago en el continente Región Metropolitana en el contexto nacional Chile en el Contexto Sudamericano Superficie continental: 816.260 Km2 Capital: Santiago, ubicada en la Región Metropolitana Superficie territorio antártico: 1.250.000 km2 Población: 15.116.435 de habitantes (aprox.) Superficie Región Metropolitana: 15.403 km2 Regiones: 13 Población de la ciudad de Santiago (R M): 6.061.185. Santiago Metropolitano Evolución 1541 1600 1900 1920 1940 1952 1960 1970 1980 1996 2004 Antecedentes generales 2005 COMUNA DE SANTIAGO Superficie : 2.320 há. QU ILI CU RA HUECHURABA VI TAC URA Población : 200.792 hab. CONCHALI RENCA Densidad : 99.5 hab/há. RECOLETA LAS CONDES INDEPENDENCIA CERRO NAVIA Población flotante: 1.800.000 QUINTA NORMAL PR OVID EN CI A PU DAH UEL LO PRADO LA REI NA SANTIAGO CENTRO FINANCIERO Y NUNOA EST C ENTR AL COMERCIAL PENALOLEN P AGUIRRE C MACUL SAN JOAQUIN Sede de las casas matrices de CERRILLOS MAIPU SAN M IGUEL bancos y financieras y de la LO ESPEJO Bolsa de Comercio LA CISTERNA LA FLORI DA SAN RAMONLA GRANJ A - 61% colocaciones bancarias EL BOSQUE - 56% depósitos y captaciones bancarias PUENTE ALTO LA PIN TANA CENTRO DE EDUCACION SAN BERNARDO - 25% de la matrícula de educación superior a escala nacional CENTRO DE CULTURA SEDE DEL PODER POLITICO Actividades hacia la sostenibilidad Certificación Ambiental El municipio de Santiago ha trabajando desde el año 2005 en el “Sistema Nacional de Certificación Ambiental de Establecimientos Educacionales”, iniciativa impulsada por organismos del gobierno central en pos de fortalecer la educación ambiental. -

Guia Comuna Recoleta
GUÍA METODOLÓGICA «OJO CON RECOLETA» • Historia de La Chimba • Cementerio General • Iglesias Recoleta Franciscana y Recoleta Dominica • Museos Histórico Dominico y de Artes Decorativas • Iglesias La Viñita y Santa Filomena • Barrios Patronato y Bellavista • La Vega y Plaza Tirso de Molina • Regimiento Buin • Cerro Blanco y Parque Santa Mónica • Liceos Paula Jaraquemada y Valentín Letelier • Casa del Pilar y Capilla Quinta Bella • El Río Mapocho 1 DESCUBRAMOS NUESTRO ENTORNO «Ama a tu ciudad. Ella es sólo la prolongación de tu hogar, y su belleza te embellece y su fealdad te avergüenza... Haz que tu ciudad sea hermosa además de rica y justa. Ama pues sus calles, que en ningún día dejas de cruzar y, que ella, por hermosa, te ayude a sentir la vida y amarla como tu Maestro quiere que la sientas: alta y espiritual». Prosa de Gabriela Mistral A través de esta Guía Metodológica, «La Ciudad: un espacio educativo», invitamos a los profesores a usar las calles y avenidas, iglesias, museos, barrios, casas, parques, cementerios y rincones de Recoleta como un medio para entusiasmar a sus alumnos con experiencias nuevas y significativas en relación a la ciudad que habitamos. La ciudad es una insospechada herramienta pedagógica para acercarnos a lo que somos. A ella –que está al alcance de la mano– podemos recurrir para comprender los vaivenes de la econo- mía, la sociedad, la política, el arte y la cultura de cada época, para admirar nuestro patrimonio cultural y natural y para encontrarnos entre sí, como conciudadanos. Recoleta, comuna joven y llena de tradiciones a la vez, cercana al núcleo fundacional de la ciudad y, simultáneamente, trazada en la antigua Chimba, al otro lado del río Mapocho, tiene una excepcional conectividad, fuerza e ímpetu que le permiten avanzar a paso sostenido hacia un desarrollo sólido e integral. -

Ofertas 12-07-2021
ÁREA CARGO COMUNA EMPRESA VACANTES GÉNERO SUELDO TURNO Abogados Abogado/a Conchalí Centro de la Mujer Conchali - Huechuraba 1Mixto 530000Turno fijo Administrativos Digitador Recepcion y despacho Quilicura STORETEK S.A. 1Mixto 450000Turno fijo Administrativos Asistente de Cobranzas Conchalí dimerc s.a 2Mixto 350000Turno fijo Administrativos Operador Call Center por Chat - Web Conchalí Pavan Consultores 10Mixto 450000Turno Rotativo Administrativos Ejecutiva de Ventas-Telemarketing Conchalí Multi Safe 1Mujer 400000Turno fijo Alimnentación MANIPULADOR ALIMENTOS Quilicura LOS ARRAYANES SA 15Mixto 350000Turno fijo Aseo AUXILIAR DE ASEO Quilicura MADEGOM 1Mujer 385000Turno fijo Aseo Aseo y Mantención Huechuraba KiosClub American Supermarket 1Mujer 400000Turno fijo Aseo Auxiliar de Aseo Conchalí EST DMV SPA 1Hombre 450000Turno fijo Aseo Auxiliar de Aseo Quilicura Grupo Eulen 16Mixto 300000Turno fijo Aseo Auxiliar de Aseo Huechuraba DONUTS FACTORY SPA 2Mixto 350000Turno fijo Aseo Auxiliar de Aseo Estación Central Nuvas Services 2Hombre 350000Turno fijo Bodegas OPERARIO DE BODEGA Recoleta TEXBAG LTDA 3Hombre 400000Turno fijo Bodegas Operarios de Bodega Pudahuel ecr Group 20Mixto 360000Turno Rotativo Bodegas ASISTENTE DE BODEGA Quilicura STORETEK S.A. 6Mixto 450000Turno fijo Bodegas Asistentes de Logística Huechuraba KiosClub American Supermarket 3Mixto 450000Turno Rotativo Bodegas OPERARIO DE BODEGA Conchalí EST DMV SPA 7Hombre 450000Turno fijo Bodegas EMBALADOR Colina REINIKE S.A 2Hombre 450000Turno fijo Bodegas Administrativo Bodega Conchalí Servicios Lucas Blandford S.A. 1Mujer 450000Turno fijo Bodegas operador de bodega Lampa Granarolo 2Hombre 500000Turno fijo Bodegas Ayudante de Logística Quilicura Abdiel Cargo Spa 4Hombre 400000Turno fijo Bodegas Operarios/as de Bodega Quilicura SOFTYS (CMPC) 5Mixto 420000Turno Rotativo Bodegas ASISTENTE DE BODEGA Quilicura FBO SPA 2Hombre 350000Turno fijo Bodegas Operario de Bodega Renca Repuestos Caren 5Hombre 500000Turno fijo Bodegas Operador de Bodega Pudahuel. -

Plan Regulador Comunal De Quinta Normal Región Metropolitana
PLAN REGULADOR COMUNAL QUINTA NORMAL REGIÓN METROPOLITANA PLAN REGULADOR COMUNAL DE QUINTA NORMAL REGIÓN METROPOLITANA MEMORIA EXPLICATIVA TOMO I SEPTIEMBRE 2019 PLAN REGULADOR COMUNAL QUINTA NORMAL REGIÓN METROPOLITANA SEPTIEMBRE 2019 PLAN REGULADOR COMUNAL QUINTA NORMAL REGIÓN METROPOLITANA ÍNDICE DE CONTENIDOS 1 ANTECEDENTES...................................................................................................................... 1-1 1.1 ANTECEDENTES GENERALES ...................................................................................... 1-1 1.2 ANTECEDENTES HISTÓRICOS ...................................................................................... 1-4 2 ESTRUCTURA URBANA .......................................................................................................... 2-1 2.1 LA COMUNA EN EL CONTEXTO METROPOLITANO E INTERCOMUNAL .................... 2-2 2.1.1 Tendencias de desarrollo urbano y su efecto en la Comuna ........................................ 2-2 2.1.2 Obras urbanas y viales y su impacto en la Comuna ..................................................... 2-7 2.1.3 Quinta Normal y sus comunas vecinas ....................................................................... 2-11 3 ANÁLISIS DE LA NORMATIVA VIGENTE ................................................................................ 3-1 3.1 PLAN REGULADOR METROPOLITANO DE SANTIAGO ................................................ 3-1 3.1.1 Zonificación y normas ..................................................................................................