Carbohydrate Polymers 220 (2019) 247–255
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Divaricating Plants in New Zealand in Relation to Moa Browsing
GREENWOOD AND ATKINSON: DIYARICATING PLANTS AND MOA BROWSING 21 EVOLUTION OF DIVARICATING PLANTS IN NEW ZEALAND IN RELATION TO MOA BROWSING R. M. GREENWOOD' and I. A. E. ATKINSON' SUMMAR Y: New Zealand appears to be the only country where spineless, small-leaved divaricating plants make up nearly 10% of the woody flora. Climatic explanations have been advanced to &ccount for the origin of these divaricating plants. We suggest that the divergent and interl~ced branching, the woody exterior and the tough stems of these plants are adaptations evolved in response to browsing by moas. Together with a few species of much smaHer birds, moas were the only browsing vertebrates in New Zealand prior to the arrival of man. Thq divaricate habit is probably only one of several strategies evolved by plants in response to moa browsing. However, because fioas fed in a different way from mammals there is little to support the idea that introduced browsing mammals have merely replaced moas as aQ ecological factor in New Zealand. MORPHOLOGICAL FEATURES OF NEW ZEALAND difficult. The most helpful keys are those of Bulmer DIY ARICATING fLANTS (1958) and Taylor (1961), which deal specifioolly The term Hdivaricating", indicating branching at with these plants. New Zealand species found in this a wide angle, is used in New Zealand to describe the investigation to be capable of divaricating are listed many species of small-leaved woody shrubs that in Table 1. In cases where there was difficulty in have closely interlaced bra,nches. Some are the deciding whether a species should be included in juvenile stages of trees that lose the divaricate habit the table the criteria used for inclusion were (i) as they grow taUer. -

One New Endemic Plant Species on Average Per Month in New Caledonia, Including Eight More New Species from Île Art (Belep Islan
CSIRO PUBLISHING Australian Systematic Botany, 2018, 31, 448–480 https://doi.org/10.1071/SB18016 One new endemic plant species on average per month in New Caledonia, including eight more new species from Île Art (Belep Islands), a major micro-hotspot in need of protection Gildas Gâteblé A,G, Laure Barrabé B, Gordon McPherson C, Jérôme Munzinger D, Neil Snow E and Ulf Swenson F AInstitut Agronomique Néo-Calédonien, Equipe ARBOREAL, BP 711, 98810 Mont-Dore, New Caledonia. BEndemia, Plant Red List Authority, 7 rue Pierre Artigue, Portes de Fer, 98800 Nouméa, New Caledonia. CHerbarium, Missouri Botanical Garden, 4344 Shaw Boulevard, Saint Louis, MO 63110, USA. DAMAP, IRD, CIRAD, CNRS, INRA, Université Montpellier, F-34000 Montpellier, France. ET.M. Sperry Herbarium, Department of Biology, Pittsburg State University, Pittsburg, KS 66762, USA. FDepartment of Botany, Swedish Museum of Natural History, PO Box 50007, SE-104 05 Stockholm, Sweden. GCorresponding author. Email: [email protected] Abstract. The New Caledonian biodiversity hotspot contains many micro-hotspots that exhibit high plant micro- endemism, and that are facing different types and intensities of threats. The Belep archipelago, and especially Île Art, with 24 and 21 respective narrowly endemic species (1 Extinct,21Critically Endangered and 2 Endangered), should be considered as the most sensitive micro-hotspot of plant diversity in New Caledonia because of the high anthropogenic threat of fire. Nano-hotspots could also be defined for the low forest remnants of the southern and northern plateaus of Île Art. With an average rate of more than one new species described for New Caledonia each month since January 2000 and five new endemics for the Belep archipelago since 2009, the state of knowledge of the flora is steadily improving. -
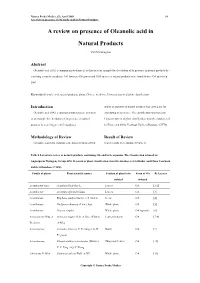
A Review on Presence of Oleanolic Acid in Natural Products
Natura Proda Medica, (2), April 2009 64 A review on presence of Oleanolic acid in Natural Products A review on presence of Oleanolic acid in Natural Products YEUNG Ming Fai Abstract Oleanolic acid (OA), a common phytochemical, is chosen as an example for elucidation of its presence in natural products by searching scientific databases. 146 families, 698 genera and 1620 species of natural products were found to have OA up to Sep 2007. Keywords Oleanolic acid, natural products, plants, Chinese medicine, Linnaeus system of plant classification Introduction and/or its saponins in natural products was carried out for Oleanolic acid (OA), a common phytochemical, is chosen elucidating its pressence. The classification was based on as an example for elucidation of its presence in natural Linnaeus system of plant classification from the databases of products by searching scientific databases. SciFinder and China Yearbook Full-text Database (CJFD). Methodology of Review Result of Review Literature search for isolation and characterization of OA Search results were tabulated (Table 1). Table 1 Literature review of natural products containing OA and/or its saponins. The classification is based on Angiosperm Phylogeny Group APG II system of plant classification from the databases of SciFinder and China Yearbook Full-text Database (CJFD). Family of plants Plant scientific names Position of plant to be Form of OA References isolated isolated Acanthaceae Juss. Acanthus illicifolius L. Leaves OA [1-2] Acanthaceae Avicennia officinalis Linn. Leaves OA [3] Acanthaceae Blepharis sindica Stocks ex T. Anders Seeds OA [4] Acanthaceae Dicliptera chinensis (Linn.) Juss. Whole plant OA [5] Acanthaceae Justicia simplex Whole plant OA saponins [6] Actinidiaceae Gilg. -

Meryta Sinclairii) on the Chickens Islands, with an Assessment of the Hypothesis That It Was Transferred There by the Maori
TANE 30, 1984 OBSERVATIONS ON PUKA (MERYTA SINCLAIRII) ON THE CHICKENS ISLANDS, WITH AN ASSESSMENT OF THE HYPOTHESIS THAT IT WAS TRANSFERRED THERE BY THE MAORI by Ross E. Beever Plant Diseases Division, DSIR, Private Bag, Auckland SUMMARY The population of the large-leaved araliad puka [Meryta sinclairii (Hook, f.) Seem.] on Lady Alice Island, Chickens Group, northern New Zealand, was estimated at c. 3 000 in 1982, a three-fold increase since 1955. Direct observations, and size class distribution studies in selected areas, indicated premature death of many apparently vigorous individuals. Various biota closely associated with puka were recorded including vertebrates, arthropods, molluscs, vascular plants, liverworts, lichens, fungi, and an alga. Premature death is attributed to root disease, tentatively assigned to Phytophthora cinnamomi Rands. The hypothesis that puka was transferred to the Hen and Chickens Islands from the Three Kings Islands by the Maori is discussed, and the possibility that it was once present on the Poor Knights Islands, but was exterminated there during Maori occupancy, is suggested. INTRODUCTION The pioneer botanist Rev. William Colenso wrote, in a letter dated 1839, of a strange tree with ' very large leaves large enough (so say natives) to wrap a cod fish in! This summer (D.V.) I shall be in the neighbourhood and then I'll get it.' (Bagnall and Petersen 1948). Puka [Meryta sinclairii (Hook.f.) Seem.], was eventually described from a solitary tree he located being cultivated by local Maoris on the mainland at Whangaruru Harbour. It is indeed large-leaved with the oblong lamina reaching c. 50 x 20 cm on petioles up to c. -

An Account of Meryta Sinclairii (Pukanui) on Marotiri Island
Immediately above this is a distinct belt of Xiphophora — beaches (Station 4) show a quite different zonation. On a peculiarly long and rather slender form which is either these platforms Hormosira banksii is very well developed a distinct species or an (marked) ecological variant. and Ulva lactuca is common. The pink basal structures Interrupting this brown belt in very, exposed places, as of Corallinas axe. abundant in more exposed places and on points and islets, are pure patches of the red algae, the erect calcareous thallus of this species frequently Pachymenia himantophora. dominates in the mtertidal pools. On shaded sides of the reefs Codium adhaerens is very common, while on exposed THE EAST COAST (STATION 2) rock faces (especially above the Xiphophora belt) Apophloea An interesting feature of the moderately exposed situations sinclairii is abundant. The oyster Saxostrea also occurs existing on the East Coast is the presence of Carpophyllum on these upper rocks of the supra-littoral fringe. Nerita maschalocarpum and C. elongatum growing together. The is conspicuous at the highest levels immediately in front C. elongatum dominates in the 'surge region' (lower-mid- of the narrow, moderately steep, shingle beach. littoral and upper infra-littoral fringe) whilst C. maschalo- carpum dominates below low tide mark in the infra- In conclusion it can be said that the zonation pattern littoral fringe proper. The presence of C. elongatum on of the Chickens appears to corrsepond closely with that the East Coast was at first puzzling, as this coastline is recognised so far by observers on other parts of the north• sheltered from the north by a low' reef and from the east east coastline of New Zealand. -

The Island Rule and Its Application to Multiple Plant Traits
The island rule and its application to multiple plant traits Annemieke Lona Hedi Hendriks A thesis submitted to the Victoria University of Wellington in partial fulfilment of the requirements for the degree of Master of Science in Ecology and Biodiversity Victoria University of Wellington, New Zealand 2019 ii “The larger the island of knowledge, the longer the shoreline of wonder” Ralph W. Sockman. iii iv General Abstract Aim The Island Rule refers to a continuum of body size changes where large mainland species evolve to become smaller and small species evolve to become larger on islands. Previous work focuses almost solely on animals, with virtually no previous tests of its predictions on plants. I tested for (1) reduced floral size diversity on islands, a logical corollary of the island rule and (2) evidence of the Island Rule in plant stature, leaf size and petiole length. Location Small islands surrounding New Zealand; Antipodes, Auckland, Bounty, Campbell, Chatham, Kermadec, Lord Howe, Macquarie, Norfolk, Snares, Stewart and the Three Kings. Methods I compared the morphology of 65 island endemics and their closest ‘mainland’ relative. Species pairs were identified. Differences between archipelagos located at various latitudes were also assessed. Results Floral sizes were reduced on islands relative to the ‘mainland’, consistent with predictions of the Island Rule. Plant stature, leaf size and petiole length conformed to the Island Rule, with smaller plants increasing in size, and larger plants decreasing in size. Main conclusions Results indicate that the conceptual umbrella of the Island Rule can be expanded to plants, accelerating understanding of how plant traits evolve on isolated islands. -
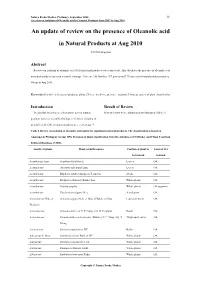
An Update of Review on the Presence of Oleanolic Acid in Natural Products at Aug 2010
Natura Proda Medica, Prelimary, September 2010 11 A review on isolation of Oleanolic acid in Natural Products from 2007 to Aug 2010 An update of review on the presence of Oleanolic acid in Natural Products at Aug 2010 YEUNG Ming Fai Abstract Reviews on isolation of oleanolic acid (OA) in natural products were carried out. This elucidates the presence of oleanolic acid in natural products based on scientific findings. There are 158 families, 767 genera and 1710 species of natural products isolated OA up to Aug 2010. Keywords Oleanolic acid, natural products, plants, Chinese medicine, presence, isolation, Linnaeus system of plant classification Introduction Result of Review To elucidate the presence of oleanolic acid in natural Review results were collaborated and tabulated (Table 1). products based on scientific findings, reviews on isolation of oleanolic acid (OA) in natural products were carried out 1-2. Table 1 Review on isolation of oleanolic acid and/or its saponins in natural products. The classification is based on Angiosperm Phylogeny Group APG II system of plant classification from the databases of SciFinder and China Yearbook Full-text Database (CJFD). Family of plants Plant scientific names Position of plant to Form of OA be isolated isolated Acanthaceae Juss. Acanthus illicifolius L. Leaves OA Acanthaceae Avicennia officinalis Linn. Leaves OA Acanthaceae Blepharis sindica Stocks ex T. Anders Seeds OA Acanthaceae Dicliptera chinensis (Linn.) Juss. Whole plants OA Acanthaceae Justicia simplex Wholeplants OAsaponins Acanthaceae Gendarussa vulgaris Nees Aerial parts OA Actinidiaceae Gilg. et Actinidia arguta (Sieb. et Zucc.) Planch. ex Miq. Leaves or stems OA Werderm. Actinidiaceae Actinidia deliciosa C. -

Curriculum Vitae
Updated July 15, 2021 Curriculum Vitae GREGORY M. PLUNKETT ADDRESS: New York Botanical Garden Phone: (718) 817-8179 2900 Southern Blvd. FAX: (718) 817-8101 Bronx, NY 10458-5126 e-mail: [email protected] BIRTH DATE: February 21, 1965. Bayonne, New Jersey, USA EDUCATION: Ph.D. (Botany), Washington State University (D.E. Soltis, advisor). 1994. M.A. (Biology), The College of William and Mary in Virginia (G.W. Hall, advisor). 1990. B.S. (Biology), The College of William and Mary in Virginia. 1987. CURRENT POSITION: Director & Curator, Program for Molecular Systematics, New York Botanical Garden. PAST FACULTY POSITIONS Professor of Biology, Virginia Commonwealth University. 2008–2009. Associate Professor of Biology, Virginia Commonwealth University. 2002–2008. Assistant Professor of Biology, Virginia Commonwealth University. 1996–2002. CURRENT AFFILIATED POSITIONS: Affiliate Professor of Biology, Virginia Commonwealth University. 2009–present. Adjunct Professor of Plant Sciences, The Graduate Center, City University of New York. 2009–present. Adjunct Faculty, Fordham University. 2014–present. Research Associate, Missouri Botanical Garden, St. Louis. 2002–present. OTHER PROFESSIONAL EXPERIENCE: Curator, Herbarium of Virginia Commonwealth University (VCU). 1996–2009. Graduate Faculty, Molecular Biology and Genetics Program, VCU. 1998–2009. Fellow, Center for the Study of Biological Complexity, VCU. 2002–2009. Visiting Curator/Professeur, Muséum National d’Histoire Naturelle, Paris. 2004, 2005. Visiting Scientist, University of the South Pacific, -

Kea (Nestor Notabilis) Care Manual
Kea (Nestor notabilis) CARE MANUAL CREATED BY THE AZA Kea Species Survival Plan® Program IN ASSOCIATION WITH THE AZA Parrot Taxon Advisory Group Kea (Nestor notabilis) Care Manual Kea (Nestor notabilis) Care Manual Published by the Association of Zoos and Aquariums in collaboration with the AZA Animal Welfare Committee Formal Citation: AZA Kea Species Survival Plan (Nestor notabilis). (2020). Kea Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: July 1, 2019 Kea (Nestor notabilis) Care Manual Coordinator: Kimberly Klosterman, Cincinnati Zoo & Botanical Garden, Senior Avian Keeper, Kea SSP Vice Coordinator Authors and Significant Contributors: Krista Adlehart CRM, Woodland Park Zoo, Animal Management Registrar Amanda Ardente NVM, PhD, Walt Disney World, University of Florida, Nutrition Fellow Jackie Bray, MA Zoology CPBT-KA, Raptor Incorporated, Associate Director Cassandre Crawford MM, Northwest Local School District, Orchestra Director, Kea SSP Volunteer Thea Etchells, Denver Zoo, Bird Keeper Linda Henry, Board Member of Zoological Lighting Institute, SeaWorld San Diego Phillip Horvey, Sedgwick County Zoo, Senior Zookeeper, Masked Lapwing SSP Coordinator and Studbook Keeper Cari Inserra, San Diego Zoo, Lead Animal Trainer Kimberly Klosterman, Cincinnati Zoo & Botanical Garden, Senior Avian Keeper, Kea Care Manual Coordinator, Vice Coordinator Kea SSP Program Jessica Meehan, Denver Zoo, Bird Keeper, Kea SSP Coordinator and Studbook Keeper Jennifer Nollman DVM, Cincinnati Zoo & Botanical Garden, Associate Veterinarian Catherine Vine, Philadelphia Zoo, Avian Keeper Reviewers: Raoul Schwing PhD, Head of Kea Lab & Infrastructure Project Manager, Messerli Research Institute, University of Vienna, AU Tamsin Orr-Walker, BAAT, Co-founder, Trustee & Chair of Kea Conservation Trust, South Island Community Engagement Coordinator, NZ Nigel Simpson, EAZA Kea EEP Coordinator, Head of Operations, Wild Place Project, Bristol Zoological Society, UK Dr.rer.nat Gyula K. -

New Zealand Plants in Australian Gardens Stuart Read
New Zealand Plants in Australian Gardens Stuart Read Abstract: (11.6.2013): Raised in a large New Zealand garden full of native trees, plant lover Stuart Read was perhaps hard-wired to notice kiwi plants in Australian gardens. Over time he's pieced together a pattern of waves of fashion in their planting and popularity, reflecting scientific and horticultural expansionism, commercial and familial networks and connections across the Tasman. Stuart will examine a range of NZ plants found in old and younger Australian gardens, try to tease out some of the means by which they got here and why they remain popular. No cabbage, This constellation of asterisks Slaps and rustles Its tough tatters In the brisk breeze; Whispers of times past And ancient histories (Barbara Mitcalfe’s poem, ‘Ti Kouka’ (cabbage tree) catches well the distinctive skyline profile of this ubiquitous New Zealand export (in Simpson, 2000, 213) Introduction / overview New Zealand gardens have been introduced to and cultivated in Australian gardens from early in their ‘discovery’, trade and exchanges between the two colonies. Australian and other explorers, botanists, nurserymen, New Zealand settlers and others searched New Zealand’s coasts and bush, bringing plants into cultivation, export and commerce from early in the settlement’s colonization. New Zealand plants have had their ‘vogue’ periods, including as: A) - Economic plants (various timbers, kauri gum for shellacs and jewellery; flax for fibre, rope, cloth; greens for scurvy; poroporo for the contraceptive ‘the pill’); B) - Exotic ornamental imports into Australian gardens and beyond to English and European conservatories (and some warmer, southern) gardens and parks; C) - Depicted or carved as subjects of botanical and other artworks, commercial commodities. -

Twenty Years of Providing Free Plants in an Urban New Zealand Setting; What Affects Community Participation and Planting Success?
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by ResearchArchive at Victoria University of Wellington TWENTY YEARS OF PROVIDING FREE PLANTS IN AN URBAN NEW ZEALAND SETTING; WHAT AFFECTS COMMUNITY PARTICIPATION AND PLANTING SUCCESS? BY PAUL BERENTSON A thesis submitted to the Victoria University of Wellington in fulfilment of the requirements for the degree of Master of Science Victoria University of Wellington 2013 ABSTRACT An urban greening programme in Wellington, New Zealand providing free plants to city residents was evaluated with the following objectives: 1. To assess the levels of plant survival after five, ten, and fifteen years and determine factors contributing to observed survival; 2. To investigate factors influencing participation in the programme; 3. To quantify the some of the socioeconomic factors relating to programme participants and environmental factors relating to sites. Data were collected from a combination of council records, site surveys and postal questionnaire surveys. The study found that plant survival was generally poor, but was mainly influenced by indigeneity of the plants. Contrary to many theories of exotic invasiveness, New Zealand native plants were 4.3 times more likely to survive than exotic plants. Site based effects were not found to influence survival significantly; nor were specific plant traits, or year of planting. A small sample of these sites was matched to questionnaire responses and it was found that length of residence by programme participants increased the performance of the best model indigeneity, indicating that increasing length of residence was a predictor of better survival of plantings. The questionnaire respondents included both those who had participated in the programme and those who had not. -

Co-Extinction of Mutualistic Species – an Analysis of Ornithophilous Angiosperms in New Zealand
DEPARTMENT OF BIOLOGICAL AND ENVIRONMENTAL SCIENCES CO-EXTINCTION OF MUTUALISTIC SPECIES An analysis of ornithophilous angiosperms in New Zealand Sandra Palmqvist Degree project for Master of Science (120 hec) with a major in Environmental Science ES2500 Examination Course in Environmental Science, 30 hec Second cycle Semester/year: Spring 2021 Supervisor: Søren Faurby - Department of Biological & Environmental Sciences Examiner: Johan Uddling - Department of Biological & Environmental Sciences “Tui. Adult feeding on flax nectar, showing pollen rubbing onto forehead. Dunedin, December 2008. Image © Craig McKenzie by Craig McKenzie.” http://nzbirdsonline.org.nz/sites/all/files/1200543Tui2.jpg Table of Contents Abstract: Co-extinction of mutualistic species – An analysis of ornithophilous angiosperms in New Zealand ..................................................................................................... 1 Populärvetenskaplig sammanfattning: Samutrotning av mutualistiska arter – En analys av fågelpollinerade angiospermer i New Zealand ................................................................... 3 1. Introduction ............................................................................................................................... 5 2. Material and methods ............................................................................................................... 7 2.1 List of plant species, flower colours and conservation status ....................................... 7 2.1.1 Flower Colours .............................................................................................................