Morphological Analysis of Palmaris Longus Muscle and Its Anatomic Variations: a Cadaveric Study in North India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study on the Absence of Palmaris Longus in a Multi-Racial Population
108472 NV-OA7 pg26-28.qxd 11/05/2007 05:02 PM Page 26 (Black plate) Malaysian Orthopaedic Journal 2007 Vol 1 No 1 SA Roohi, etal A Study on the Absence of Palmaris Longus in a Multi- racial Population SA Roohi, MS (Ortho) (UKM), L Choon-Sian, MD (UKM), A Shalimar, MS (Ortho) (UKM), GH Tan, MS (Ortho) (UKM), AS Naicker, M Med Rehab (UM) Hospital Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia ABSTRACT Most standard textbooks of hand surgery quote the prevalence of absence of palmaris longus at around 15%3-5. Palmaris longus is a dispensable muscle with a long tendon However, this figure varies considerably in different ethnic which is very useful in reconstructive surgery. It is absent groups. A study by Thompson et al6 on 300 Caucasian 2.8 to 24% of the population depending on the race/ethnicity subjects found that palmaris longus was absent unilaterally in studied. Four hundred and fifty healthy subjects (equally 16%, and bilaterally in 9% of the study sample for an overall distributed among Malaysia’s 3 major ethnic groups) were prevalence of absence of 24%. Similarly, George7 noted on clinically examined for the presence or absence of palmaris 276 cadavers of European descent that its absence was 13% longus. This tendon was found to be absent unilaterally in unilaterally, 8.7% bilaterally for an overall absence of 15.2%. 6.4% of study subjects, and bilaterally in 2.9% of study Another cadaveric study by Vanderhooft8 in Seattle, USA participants. Malays have a high prevalence of palmaris reported its overall absence to be 12%. -

Median Nerve Compression at Pronator Teres
1 Median Nerve Compression at Pronator Teres Surgical Indications and Considerations Anatomical Considerations: The median nerve and brachial artery travel together down the arm. Therefore, one must be very careful not to interfere with either the median nerve or the brachial artery, especially when conducting surgical procedures. In the area of the pronator teres, there are many tendons as well. It is important to identify, as much as possible, the correct site of compression. Pathogenesis: The median nerve can get entrapped or compressed by several structures in the arm. The pronator teres muscle is the most common. Others entrapment sites include the flexor digitorum superficialis arch, the lacertus fibrosis (bicipital aponeurosis), and ligament of Struthers (frequency occurs in that order). For compression of the median nerve at the pronator teres and flexor digitorum superficialis, the cause is almost always due to hypertrophy of the respected muscle. This hypertrophy is from quick, forceful and repeated movements to the involved muscle. Examples include a carpenter or a baseball batter. As the muscle hypertrophies, the signal from the median nerve is diminished resulting in paresthesias in the median nerve distribution (lateral arm and hand) distal to the site of compression. Pain in the volar part of the forearm, often aggravated by repetitive supination and pronation, is a common symptom of pronator involvement. Another indicator is forearm pain with the compression of muscle such as pain in the volar part of the forearm implicating pronator teres. Onset is typically insidious and diagnosis is usually delayed 9 months to 2 years. Epidemiology: Pronator teres syndrome is the second most common cause of median nerve compression behind carpal tunnel syndrome. -

Early Passive Motion After Surgery
www.western -ortho.com www.denvershoulder.com Early Passive Motion after Shoulder Surgery Passive motion involves someone else moving the affected arm through the motion described. Or, in the case of elbow flexion/extension, you can use your opposite (non-affected arm) to move through the motion. Do 5 repetitions of each stretch 3 times per day. When you feel a slight ‘tightness’ with your arm in the position diagrammed, hold that position for 30 seconds. If lying down is difficult, the stretches can be done while seated. Shoulder Flexion Support arm at the wrist and elbow. With the thumb pointed forward, gently bring the arm up and forward then back to the side. Shoulder Abduction Support arm at wrist and elbow. With the thumb pointed away from the body and palm up, gently bring the arm out to the side. www.western -ortho.com www.denvershoulder.com Shoulder Internal/External Rotation Support arm at wrist and elbow. With the elbow at the side and bent to a 90 degree angle, gently rotate the hand away from the body down toward the table the individual is lying on. Elbow Flexion/Extension Forearm Pronation/Supination Grasp the wrist of your affected arm with your unaffected With your elbow and forearm supported on a table, hand. With your affected elbow against your side and your gently turn forearm so your palm is down, then turn palm up, gently bend and straighten your elbow. forearm so your palm is up. This can be done actively (without assistance from your other hand). . -

Pronator Syndrome: Clinical and Electrophysiological Features in Seven Cases
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.39.5.461 on 1 May 1976. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry, 1976, 39, 461-464 Pronator syndrome: clinical and electrophysiological features in seven cases HAROLD H. MORRIS AND BRUCE H. PETERS From the Department ofNeurology, University of Texas Medical Branch, Galveston, Texas, USA SYNOPSIS The clinical and electrophysiological picture of seven patients with the pronator syndrome is contrasted with other causes ofmedian nerve neuropathy. In general, these patients have tenderness over the pronator teres and weakness of flexor pollicis longus as well as abductor pollicis brevis. Conduction velocity of the median nerve in the proximal forearm is usually slow but the distal latency and sensory nerve action potential at the wrist are normal. Injection of corticosteroids into the pronator teres has produced relief of symptoms in a majority of patients. Protected by copyright. In the majority of isolated median nerve dys- period 101 cases of the carpal tunnel syndrome functions the carpal tunnel syndrome is appropri- and the seven cases of the pronator syndrome ately first suspected. The median nerve can also reported here were identified. Median nerve be entrapped in the forearm giving rise to a conduction velocity determinations were made on similar picture and an erroneous diagnosis. all of these patients. The purpose of this report is to draw full attention to the pronator syndrome and to the REPORT OF CASES features which allow it to be distinguished from Table 1 provides clinical details of seven cases of the median nerve entrapment at other sites. -

M1 – Muscled Arm
M1 – Muscled Arm See diagram on next page 1. tendinous junction 38. brachial artery 2. dorsal interosseous muscles of hand 39. humerus 3. radial nerve 40. lateral epicondyle of humerus 4. radial artery 41. tendon of flexor carpi radialis muscle 5. extensor retinaculum 42. median nerve 6. abductor pollicis brevis muscle 43. flexor retinaculum 7. extensor carpi radialis brevis muscle 44. tendon of palmaris longus muscle 8. extensor carpi radialis longus muscle 45. common palmar digital nerves of 9. brachioradialis muscle median nerve 10. brachialis muscle 46. flexor pollicis brevis muscle 11. deltoid muscle 47. adductor pollicis muscle 12. supraspinatus muscle 48. lumbrical muscles of hand 13. scapular spine 49. tendon of flexor digitorium 14. trapezius muscle superficialis muscle 15. infraspinatus muscle 50. superficial transverse metacarpal 16. latissimus dorsi muscle ligament 17. teres major muscle 51. common palmar digital arteries 18. teres minor muscle 52. digital synovial sheath 19. triangular space 53. tendon of flexor digitorum profundus 20. long head of triceps brachii muscle muscle 21. lateral head of triceps brachii muscle 54. annular part of fibrous tendon 22. tendon of triceps brachii muscle sheaths 23. ulnar nerve 55. proper palmar digital nerves of ulnar 24. anconeus muscle nerve 25. medial epicondyle of humerus 56. cruciform part of fibrous tendon 26. olecranon process of ulna sheaths 27. flexor carpi ulnaris muscle 57. superficial palmar arch 28. extensor digitorum muscle of hand 58. abductor digiti minimi muscle of hand 29. extensor carpi ulnaris muscle 59. opponens digiti minimi muscle of 30. tendon of extensor digitorium muscle hand of hand 60. superficial branch of ulnar nerve 31. -

A STUDY of PALMARIS LONGUS MUSCLE: ITS ANATOMIC VARIATIONS with EMBRYOLOGICAL SIGNIFICANCE and CLINICAL IMPORTANCE Sunitha
International Journal of Anatomy and Research, Int J Anat Res 2018, Vol 6(2.2):5222-27. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2018.161 A STUDY OF PALMARIS LONGUS MUSCLE: ITS ANATOMIC VARIATIONS WITH EMBRYOLOGICAL SIGNIFICANCE AND CLINICAL IMPORTANCE Sunitha. R 1, Prathap Kumar J *2. 1 Assistant Professor, Department of Anatomy, Sambhram Institute of Medical Science and Research, KGF, Kolar, Karnataka, India. *2 Assistant Professor, Department of Anatomy, Ramaiah Medical College, Bangalore, Karnataka, India. ABSTRACT Background: In the present study, variations in the Palmaris longus and the clinical implications of these are discussed. Aim: To study the variations in the Palmaris longus and to discuss the embryological basis, clinical and surgical implications of these variations. Materials and Methods: This study was conducted in Department of Anatomy of Hassan Institute of Medical Science, Hassan, Dr B.R.Ambedkar Medical college, Bangalore and Sri Devaraj Urs Academy of Higher Education and Research,Tamaka, Kolar. Thirty formalin fixed cadavers (60 upper limbs); 25 males & 5 female cadavers were dissected for the study and it was conducted over a period of three years, i.e., from 2011-2014. The cadavers with visible trauma, pathology or prior surgeries were excluded from the study. Routine dissection of the upper limb was carried out following the Cunnigham’s Manual of Practical Anatomy. During the dissection of the anterior compartment of forearm, the Palmaris longus muscle was identified & carefully dissected. At first, the origin was confirmed and then, it was traced towards its insertion. Any variations found were noted and photographed. -

Anatomical Study of the Branch of the Palmaris Longus Muscle for Its Transfer to the Posterior Interosseous Nerve
Int. J. Morphol., 37(2):626-631, 2019. Anatomical Study of the Branch of the Palmaris Longus Muscle for its Transfer to the Posterior Interosseous Nerve Estudio Anatómico del Ramo del Músculo Palmar Largo para su Transferencia al Nervio Interóseo Posterior Edie Benedito Caetano1; Luiz Angelo Vieira1; Maurício Benedito Ferreira Caetano2; Cristina Schmitt Cavalheiro3; Marcel Henrique Arcuri3 & Luís Cláudio Nascimento da Silva Júnior3 CAETANO, E. B.; VIEIRA, L. A.; FERREIRA, C. M. B.; CAVALHEIRO, C. S.; ARCURI, M. H. & SILVA JÚNIOR, L. C. N. Anatomical study of the branch of the palmaris longus muscle for its transfer to the posterior interosseous nerve. Int. J. Morphol., 37(2):626-631, 2019. SUMMARY: The objective of the study was to evaluate the anatomical characteristics and variations of the palmaris longus nerve branch and define the feasibility of transferring this branch to the posterior interosseous nerve without tension. Thirty arms from 15 adult male cadavers were dissected after preparation with 20 % glycerin and formaldehyde intra-arterial injection. The palmaris longus muscle (PL) received exclusive innervation of the median nerve in all limbs. In most it was the second muscle of the forearm to be innervated by the median nerve. In 5 limbs the PL muscle was absent. In 5 limbs we identified a branch without sharing branches with other muscles. In 4 limbs it shared origin with the pronator teres (PT), in 8 with the flexor carpi radialis (FCR), in 2 with flexor digitorum superficialis (FDS), in 4 shared branches for the PT and FCR and in two with PT, FCR, FDS. The mean length was (4.0 ± 1.2) and the thickness (1.4 ± 0.6). -

Self Range of Motion Exercises for Arm and Hand
Self-Range of Motion Exercises for the Arm and Hand After a stroke, it is important to do the exercises in this handout for your affected arm and hand. You can do them on your own by using your unaffected arm and hand. These gentle movements are called “self-range of motion” exercises, and they help to maintain your movement, prevent stiffness, improve blood flow, and increase awareness of your affected arm and hand. Complete the exercises slowly and do not force movements. Stop if you feel pain. If you have any questions or concerns, please contact your Occupational Therapist: _______________________________ Do the exercises in this handout _____ times each day. Page - 1 Self-range of motion exercises for the arm and hand 1. Shoulder: Forward Arm Lift Interlock your fingers, or hold your wrist. With your elbows straight and thumbs facing the ceiling, lift your arms to shoulder height. Slowly lower your arms to starting position. Hold for ____ seconds. Repeat ____ times. Page - 2 Self-range of motion exercises for the arm and hand 2. Shoulder: “Rock the Baby” Stretch Hold your affected arm by supporting the elbow, forearm and wrist (as if cradling a baby). Slowly move your arms to the side, away from your body, lifting to shoulder height. Repeat this motion in the other direction. Slowly rock your arms side-to-side, and keep your body from turning. Repeat ____ times. Page - 3 Self-range of motion exercises for the arm and hand 3. Shoulder: Rotation Stretch Interlock your fingers, or hold your wrist. With your elbows bent at 90 degrees, keep your affected arm at your side. -

Morphological Study of Palmaris Longus Muscle
International INTERNATIONAL ARCHIVES OF MEDICINE 2017 Medical Society SECTION: HUMAN ANATOMY Vol. 10 No. 215 http://imedicalsociety.org ISSN: 1755-7682 doi: 10.3823/2485 Humberto Ferreira Morphological Study of Palmaris Arquez1 Longus Muscle ORIGINAL 1 University of Cartagena. University St. Thomas. Professor Human Morphology, Medicine Program, University of Pamplona. Morphology Laboratory Abstract Coordinator, University of Pamplona. Background: The palmaris longus is one of the most variable muscle Contact information: in the human body, this variations are important not only for the ana- tomist but also radiologist, orthopaedic, plastic surgeons, clinicians, Humberto Ferreira Arquez. therapists. In view of this significance is performed this study with Address: University Campus. Kilometer the purpose to determine the morphological variations of palmaris 1. Via Bucaramanga. Norte de Santander, longus muscle. Colombia. Suramérica. Tel: 75685667-3124379606. Methods and Findings: A total of 17 cadavers with different age groups were used for this study. The upper limbs region (34 [email protected] sides) were dissected carefully and photographed in the Morphology Laboratory at the University of Pamplona. Of the 34 limbs studied, 30 showed normal morphology of the palmaris longus muscle (PL) (88.2%); PL was absent in 3 subjects (8.85% of all examined fo- rearm). Unilateral absence was found in 1 male subject (2.95% of all examined forearm); bilateral agenesis was found in 2 female subjects (5.9% of all examined forearm). Duplicated palmaris longus muscle was found in 1 male subject (2.95 % of all examined forearm). The palmaris longus muscle was innervated by branches of the median nerve. The accessory palmaris longus muscle was supplied by the deep branch of the ulnar nerve. -
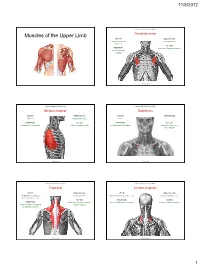
Muscles of the Upper Limb.Pdf
11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles of the Upper Limb Pectoralis minor ORIGIN: INNERVATION: anterior surface of pectoral nerves ribs 3 – 5 ACTION: INSERTION: protracts / depresses scapula coracoid process (scapula) (Anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Serratus anterior Subclavius ORIGIN: INNERVATION: ORIGIN: INNERVATION: ribs 1 - 8 long thoracic nerve rib 1 ---------------- INSERTION: ACTION: INSERTION: ACTION: medial border of scapula rotates scapula laterally inferior surface of scapula stabilizes / depresses pectoral girdle (Lateral view) (anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Trapezius Levator scapulae ORIGIN: INNERVATION: ORIGIN: INNERVATION: occipital bone / spinous accessory nerve transverse processes of C1 – C4 dorsal scapular nerve processes of C7 – T12 ACTION: INSERTION: ACTION: INSERTION: stabilizes / elevates / retracts / upper medial border of scapula elevates / adducts scapula acromion / spine of scapula; rotates scapula lateral third of clavicle (Posterior view) (Posterior view) 1 11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles Moving Arm Rhomboids Pectoralis major (major / minor) ORIGIN: INNERVATION: ORIGIN: INNERVATION: spinous processes of C7 – T5 dorsal scapular nerve sternum / clavicle / ribs 1 – 6 dorsal scapular nerve INSERTION: ACTION: INSERTION: ACTION: medial border of scapula adducts / rotates scapula intertubucular sulcus / greater tubercle flexes / medially rotates / (humerus) adducts -
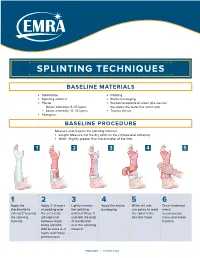
Splinting Techniques
SPLINTING TECHNIQUES BASELINE MATERIALS l Stockinette l Padding l Splinting material l Elastic bandaging l Plaster l Bucket/receptacle of water (the warmer — Upper extremity: 8–10 layers the water, the faster the splint sets) — Lower extremity: 10–12 layers l Trauma shears l Fiberglass BASELINE PROCEDURE Measure and prepare the splinting material. l Length: Measure out the dry splint on the contralateral extremity l Width: Slightly greater than the diameter of the limb 1 2 3 4 5 1 2 3 4 5 6 Apply the Apply 2–3 layers Lightly moisten Apply the elastic While still wet, Once hardened, stockinette to of padding over the splinting bandaging. use palms to mold check extend 2" beyond the area to be material. Place it the splint to the neruovascular the splinting splinted and and fold the ends desired shape. status and motor material. between digits of stockinette function. being splinted. over the splinting Add an extra 2–3 material. layers over bony prominences. EMRA.ORG | 972.550.0920 POSTERIOR LONG ARM VOLAR SPLINT SPLINT INDICATIONS INDICATIONS l Olecranon fractures l Soft tissue injuries of the hand and wrist l Humerus fractures l Carpal bone fractures l Radial head and neck fractures l 2nd–5th metacarpal head fractures CONSTRUCTION CONSTRUCTION l Start at posterior proximal arm l Start at palm at the metacarpal heads l Down the ulnar forearm l Down the volar forearm l End at the metacarpophalangeal joints l End at distal forearm APPLICATION APPLICATION l Cut hole in stockinette for thumb l Cut hole in stockinette for thumb l Elbow at 90º -

The Impact of Palmaris Longus Muscle on Function in Sports: an Explorative Study in Elite Tennis Players and Recreational Athletes
Journal of Functional Morphology and Kinesiology Article The Impact of Palmaris Longus Muscle on Function in Sports: An Explorative Study in Elite Tennis Players and Recreational Athletes Julie Vercruyssen 1,*, Aldo Scafoglieri 2 and Erik Cattrysse 2 1 Faculty of Physical Education and Physiotherapy, Master of Science in Manual therapy, Vrije Universiteit Brussel, Laarbeeklaan 103, 1090 Brussels, Belgium 2 Faculty of Physical Education and Physiotherapy, Department of Experimental Anatomy, Vrije Universiteit Brussel, Laarbeeklaan 103, 1090 Brussels, Belgium; [email protected] (A.S.); [email protected] (E.C.) * Correspondence: [email protected]; Tel.: +32-472-741-808 Academic Editor: Giuseppe Musumeci Received: 21 February 2016; Accepted: 24 March 2016; Published: 13 April 2016 Abstract: The Palmaris longus muscle can be absent unilateral or bilateral in about 22.4% of human beings. The aim of this study is to investigate whether the presence of the Palmaris longus muscle is associated with an advantage to handgrip in elite tennis players compared to recreational athletes. Sixty people participated in this study, thirty elite tennis players and thirty recreational athletes. The presence of the Palmaris longus muscle was first assessed using different tests. Grip strength and fatigue resistance were measured by an electronic hand dynamometer. Proprioception was registered by the Flock of Birds electromagnetic tracking system. Three tests were set up for measuring proprioception: joint position sense, kinesthesia, and joint motion sense. Several hand movements were conducted with the aim to correctly reposition the joint angle. Results demonstrate a higher presence of the Palmaris longus muscle in elite tennis players, but this was not significant.