White-Chinned Petrel Distribution Abudance & Connectivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proposal for Inclusion of the Antipodean Albatross in Appendix I
CONVENTION ON UNEP/CMS/COP13/Doc. 27.1.7 MIGRATORY 25 September 2019 Original: English SPECIES 13th MEETING OF THE CONFERENCE OF THE PARTIES Gandhinagar, India, 17 - 22 February 2020 Agenda Item 27.1 PROPOSAL FOR THE INCLUSION OF THE ANTIPODEAN ALBATROSS (Diomedea antipodensis) ON APPENDIX I OF THE CONVENTION Summary: The Governments of New Zealand, Australia and Chile have submitted the attached proposal for the inclusion of the Antipodean albatross (Diomedea antipodensis) on Appendix I of CMS. The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CMS Secretariat (or the United Nations Environment Programme) concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. UNEP/CMS/COP13/Doc. 27.1.7 PROPOSAL FOR INCLUSION OF THE ANTIPODEAN ALBATROSS (Diomedea antipodensis) ON APPENDIX I OF THE CONVENTION A. PROPOSAL Inclusion of Diomedea antipodensis on the Convention on the Conservation of Migratory Species of Wild Animals (CMS) Appendix I. The current CMS Appendix II listing will remain in place. Diomedea antipodensis is classified as Endangered (IUCN) as it is undergoing a very rapid decline in population size. B. PROPONENT: Governments of New Zealand, Australia and Chile. C. SUPPORTING STATEMENT 1. Taxonomy 1.1 Class: Aves 1.2 Order: Procellariiformes 1.3 Family: Diomedeidae (albatrosses) 1.4 Genus, species or subspecies, including author and year: Diomedea antipodensis (Robertson & Warham 1992), including two subspecies: Diomedea antipodensis antipodensis and Diomedea antipodensis gibsoni 1.5 Scientific synonyms: Diomedea exulans antipodensis Diomedea antipodensis was formerly included in the wandering albatross complex (Diomedea exulans) (e.g. -

Bugoni 2008 Phd Thesis
ECOLOGY AND CONSERVATION OF ALBATROSSES AND PETRELS AT SEA OFF BRAZIL Leandro Bugoni Thesis submitted in fulfillment of the requirements for the degree of Doctor of Philosophy, at the Institute of Biomedical and Life Sciences, University of Glasgow. July 2008 DECLARATION I declare that the work described in this thesis has been conducted independently by myself under he supervision of Professor Robert W. Furness, except where specifically acknowledged, and has not been submitted for any other degree. This study was carried out according to permits No. 0128931BR, No. 203/2006, No. 02001.005981/2005, No. 023/2006, No. 040/2006 and No. 1282/1, all granted by the Brazilian Environmental Agency (IBAMA), and International Animal Health Certificate No. 0975-06, issued by the Brazilian Government. The Scottish Executive - Rural Affairs Directorate provided the permit POAO 2007/91 to import samples into Scotland. 2 ACKNOWLEDGEMENTS I was very lucky in having Prof. Bob Furness as my supervisor. He has been very supportive since before I had arrived in Glasgow, greatly encouraged me in new initiatives I constantly brought him (and still bring), gave me the freedom I needed and reviewed chapters astonishingly fast. It was a very productive professional relationship for which I express my gratitude. Thanks are also due to Rona McGill who did a great job in analyzing stable isotopes and teaching me about mass spectrometry and isotopes. Kate Griffiths was superb in sexing birds and explaining molecular methods again and again. Many people contributed to the original project with comments, suggestions for the chapters, providing samples or unpublished information, identifyiyng fish and squids, reviewing parts of the thesis or helping in analysing samples or data. -

Iucn Red Data List Information on Species Listed On, and Covered by Cms Appendices
UNEP/CMS/ScC-SC4/Doc.8/Rev.1/Annex 1 ANNEX 1 IUCN RED DATA LIST INFORMATION ON SPECIES LISTED ON, AND COVERED BY CMS APPENDICES Content General Information ................................................................................................................................................................................................................................ 2 Species in Appendix I ............................................................................................................................................................................................................................... 3 Mammalia ............................................................................................................................................................................................................................................ 4 Aves ...................................................................................................................................................................................................................................................... 7 Reptilia ............................................................................................................................................................................................................................................... 12 Pisces ................................................................................................................................................................................................................................................. -

Population Trends of Spectacled Petrels Procellaria Conspicillata and Other Seabirds at Inaccessible Island
Ryan et al.: Population trends for Spectacled Petrels at Inaccessible Island 257 POPULATION TRENDS OF SPECTACLED PETRELS PROCELLARIA CONSPICILLATA AND OTHER SEABIRDS AT INACCESSIBLE ISLAND PETER G. RYAN1*, BEN J. DILLEY1 & ROBERT A. RONCONI2 1FitzPatrick Institute of African Ornithology, DST-NRF Centre of Excellence, University of Cape Town, Rondebosch 7701, South Africa *([email protected]) 2Canadian Wildlife Service, Environment and Climate Change Canada, 45 Alderney Dr., Dartmouth, NS B2Y 2N6, Canada Received 21 March 2019, accepted 29 July 2019 ABSTRACT RYAN, P.G., DILLEY, B.J. & RONCONI, R.A. 2019. Population trends of Spectacled Petrels Procellaria conspicillata and other seabirds at Inaccessible Island. Marine Ornithology 47: 257–265. Inaccessible Island, in the Tristan da Cunha archipelago, is the sole breeding site of the Spectacled Petrel Procellaria conspicillata. The island also supports globally important populations of four threatened seabirds, as well as populations of other seabird species. A seabird monitoring protocol was established in 2004, following baseline surveys of most surface-breeding species in 1999. For the species monitored, we report population trends that are based on visits in 2009 and 2018. Populations of most monitored species appear to be stable or increasing, including three albatross species currently listed as Endangered or Critically Endangered. However, numbers of Northern Rockhopper Penguin Eudyptes moseleyi may have decreased slightly since 1999, and numbers of Antarctic Tern Sterna vittata have decreased since 1982. The population of Spectacled Petrels is estimated to be at least 30 000 pairs and continues to increase since feral pigs Sus scrofa died out on the island in the early 20th century. -

Petrelsrefs V1.1.Pdf
Introduction I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2017 & 2018 to: [email protected]. Please note that this and other Reference Lists I have compiled are not exhaustive and are best employed in conjunction with other sources. Grateful thanks to Killian Mullarney and Tom Shevlin (www.irishbirds.ie) for the cover images. All images © the photographers. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2017. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 7.3 accessed August 2017]). Version Version 1.1 (August 2017). Cover Main image: Bulwer’s Petrel. At sea off Madeira, North Atlantic. 14th May 2012. Picture by Killian Mullarney. Vignette: Northern Fulmar. Great Saltee Island, Co. Wexford, Ireland. 5th May 2008. Picture by Tom Shevlin. Species Page No. Antarctic Petrel [Thalassoica antarctica] 12 Beck's Petrel [Pseudobulweria becki] 18 Blue Petrel [Halobaena caerulea] 15 Bulwer's Petrel [Bulweria bulweri] 24 Cape Petrel [Daption capense] 13 Fiji Petrel [Pseudobulweria macgillivrayi] 19 Fulmar [Fulmarus glacialis] 8 Giant Petrels [Macronectes giganteus & halli] 4 Grey Petrel [Procellaria cinerea] 19 Jouanin's Petrel [Bulweria fallax] 27 Kerguelen Petrel [Aphrodroma brevirostris] 16 Mascarene Petrel [Pseudobulweria aterrima] 17 Parkinson’s Petrel [Procellaria parkinsoni] 23 Southern Fulmar [Fulmarus glacialoides] 11 Spectacled Petrel [Procellaria conspicillata] 22 Snow Petrel [Pagodroma nivea] 14 Tahiti Petrel [Pseudobulweria rostrata] 18 Westland Petrel [Procellaria westlandica] 23 White-chinned Petrel [Procellaria aequinoctialis] 20 1 Relevant Publications Beaman, M. -

Castaway – the Diary of Samuel Abraham Clark, Disappointment Island, 1907 (My Story) Author: Bill O’Brien
Teacher Notes Scholastic New Zealand Limited Castaway – The diary of Samuel Abraham Clark, Disappointment Island, 1907 (My Story) Author: Bill O’Brien Synopsis Samuel Clark is 13 years-old. His parents both recently died in a house fire and the only family that he has left is his 21 year-old brother Joseph. Joseph is a seaman who is based in England. Mr Harrington, a close family friend, took Samuel in after his parents’ deaths. He paid for Samuel to travel from Dunedin to Sydney where he is to board the Dundonald and work as a cabin boy to pay for his passage to England, where he will reunite with Joseph. Life aboard the Dundonald is a real eye-opener for Samuel. It takes him some time to find his sea-legs, the hours are long and Mr Smith, the ship’s steward, takes an instant dislike to him. Samuel soon adapts however and is eager to learn from the more experienced crew members and listen as they regale him with tales of their adventures at sea. The passage that the Dundonald is taking is a perilous one. The ship heads through the Southern Ocean which is known for its freezing temperatures and rough seas. Dundonald is hit by several ferocious storms and after a few days of fog, loses her bearings. Unbeknownst to the Captain and crew the Dundonald has sailed close to Disappointment Island. The Dundonald hits a sunken reef. The ship slams into the sheer rock cliffs that span the Island. The men are forced to abandon ship and many lose their lives in the process. -

Year Away by D.O.W
Year Away Coastwatching in the South Pacific: Disappointment Island from the western cliffs of Auckland Island (from: Coastwatchers by D.O.W. Hall, War History Branch, Department of Internal Affairs, Wellington, 1951). Year Away Wartime Coastwatching on the Auckland Islands, 1944 Graham Turbott The cover incorporates observer charts for ships and aircraft used by the coastwatchers (from the author’s files); photo and outline map are from Coastwatchers by D.O.W. Hall, War History Branch, Department of Internal Affairs, Wellington, 1951. These memoirs were published with the financial support of the Department of Conservation, Science & Research Unit (Manager Social, Historic and Technical: Paul Dingwall). Editing and illustration research was done by Janet Hughes; the layout was by Jeremy Rolfe; Chris Edkins designed the cover and the map. Graeme Taylor alerted the publisher to the manuscript and, with Paul Dingwall, provided factual updates. Production by DOC Science Publishing was managed by Jaap Jasperse and printing co-ordinated by Sue Wilkins. Publication was approved by the Manager, Science & Research Unit, Science Technology and Information Services, Department of Conservation, Wellington. © September 2002, Department of Conservation ISBN 0-478-22268-8 National Library of New Zealand Cataloguing-in-Publication Data Turbott, E. G. (Evan Graham) Year away : wartime coastwatching on the Auckland Islands, 1944 / Graham Turbott. ISBN 0-478-22268-8 1. Turbott, E. G. (Evan Graham) 2. New Zealand Sub-Antarctic Expedition 1941-1945. 3. Coastal surveillance—New Zealand—Auckland Islands— History. 4. Military surveillance—New Zealand—Auckland Islands— History. 5. Natural history—New Zealand—Auckland Islands. 6. Auckland Islands (N.Z.)—Surveys. -

Full Article
5 Shipwrecks and mollymawks: an account of Disappointment Island birds KATH J. WALKER* PAUL M. SAGAR GRAEME P. ELLIOTT 418 Pleasant Valley Road, RD21, Albatross Research, 549 Rocks Road, Geraldine 7991, New Zealand Nelson 7011, New Zealand PETER J. MCCLELLAND KALINKA REXER-HUBER 237 Kennington-Roslyn Bush Road, RD2, GRAHAM C. PARKER Invercargill 9872, New Zealand Parker Conservation, 126 Maryhill Terrace, Dunedin 9011, New Zealand ABSTRACT: This paper describes the birds of Disappointment Island, a small pristine island in the subantarctic Auckland Islands archipelago, from an accumulation of observations made by ornithologists during 16 visits to the island during 1907–2019. The island supports large populations of both the Auckland Island rail (Lewinia muelleri) and the Auckland Island teal (Anas aucklandica), most of the global population of the New Zealand endemic white-capped mollymawk (Thalassarche cauta steadi) – an annual average of 63,856 breeding pairs during 2009–17, an estimated 155,500 pairs of the circumpolar white-chinned petrel (Procellaria aequinoctialis), and unquantified numbers of smaller petrels. The topography and vegetation communities of the island are described, the history of visits by ornithologists to the island is outlined, and a list of bird species and their breeding status is recorded. Walker, K.J.; Elliott, G.P.; Rexer-Huber, K.; Parker, G.C.; Sagar, P.M.; McClelland, P.J. 2020. Shipwrecks and mollymawks: an account of Disappointment Island birds. Notornis 67(1): 213–245. KEYWORDS: Disappointment Island, seabirds, Auckland Island rail, white-capped mollymawk, aerial census, ground-truth Received 30 May 2019; accepted 28 August 2019 *Correspondence: [email protected] Introduction Disappointment Island Disappointment Island (50°36’S, 165°58’E, 284 The island comprises altered basaltic rocks cut by ha) lies off the rugged west coast of Auckland conspicuous white rhyolite dykes. -

Threatened Species PROGRAMME Threatened Species: a Guide to Red Lists and Their Use in Conservation LIST of ABBREVIATIONS
Threatened Species PROGRAMME Threatened Species: A guide to Red Lists and their use in conservation LIST OF ABBREVIATIONS AOO Area of Occupancy BMP Biodiversity Management Plan CBD Convention on Biological Diversity CITES Convention on International Trade in Endangered Species DAFF Department of Agriculture, Forestry and Fisheries EIA Environmental Impact Assessment EOO Extent of Occurrence IUCN International Union for Conservation of Nature NEMA National Environmental Management Act NEMBA National Environmental Management Biodiversity Act NGO Non-governmental Organization NSBA National Spatial Biodiversity Assessment PVA Population Viability Analysis SANBI South African National Biodiversity Institute SANSA South African National Survey of Arachnida SIBIS SANBI's Integrated Biodiversity Information System SRLI Sampled Red List Index SSC Species Survival Commission TSP Threatened Species Programme Threatened Species: A guide to Red Lists and their use in conservation OVERVIEW The International Union for Conservation of Nature (IUCN)’s Red List is a world standard for evaluating the conservation status of plant and animal species. The IUCN Red List, which determines the risks of extinction to species, plays an important role in guiding conservation activities of governments, NGOs and scientific institutions, and is recognized worldwide for its objective approach. In order to produce the IUCN Red List of Threatened Species™, the IUCN Species Programme, working together with the IUCN Species Survival Commission (SSC) and members of IUCN, draw on and mobilize a network of partner organizations and scientists worldwide. One such partner organization is the South African National Biodiversity Institute (SANBI), who, through the Threatened Species Programme (TSP), contributes information on the conservation status and biology of threatened species in southern Africa. -

The Grafton Wreck and Epigwaitt Hut Site, Auckland Islands Heritage Assessment
HERITAGE ASSESSMENT SERIES 1 The Grafton wreck and Epigwaitt hut site, Auckland Islands Heritage assessment Peter Petchey Peer review statement Assessment prepared by Peter Petchey, Southern Archaeology Ltd, Dunedin Date: 2014 Assessment reviewed by Neville Ritchie, Department of Conservation. Date: 18/12/2014 Cover: The hull of the Grafton, Carnley Harbour, Auckland Islands, 2003. Photo: Henk Haazen. The Heritage Assessment Series presents research funded by the New Zealand Department of Conservation (DOC). A heritage assessment is the key document used by DOC to identify the heritage values and significance of a place and, in turn, determine its management. Heritage assessments are prepared, and peer-reviewed, by heritage specialists. As they have been commissioned on an individual basis, there will be some variation in the structure of the reports that appear in the series. This report is available from the departmental website in pdf form. Titles are listed in our catalogue on the website, refer www.doc.govt.nz under Publications, then Series Unless otherwise stated, all images contained within this report remain the property of the owners and must not be reproduced in other material without their permission. © Copyright July 2016, New Zealand Department of Conservation ISSN 2463–6304 (web PDF) ISBN 978–0–478–15082–7 (web PDF) This report was prepared for publication by the Publishing Team; editing by Amanda Todd and layout by Lynette Clelland. Publication was approved by the Director, Recreation, Tourism and Heritage Unit, Department of Conservation, Wellington, New Zealand. Published by Publishing Team, Department of Conservation, PO Box 10420, The Terrace, Wellington 6143, New Zealand. -

Ships, Sailormen and Their Passengers
W E L C O M E T O T H E H O C K E N 50c Friends of the Hocken Collections B U L L E T I N N U M B E R 14 : October 1995 Ships, sailormen and their passengers UCH a richness of maritime material exists in the Hocken Library collections S that—even without the inclusion of Exploration—this Bulletin can be no more than an introduction, with the emphasis placed on New Zealand and related Australian resources. Some aspects have already been covered in earlier Bulletins, such as hydrographic charts (No.5); and relevant items are also to be found in missionaries’ papers (No.8), newspapers (No.3), political papers (No.6), surveying (No.9), and Americana (No.13). Magnet, 1840; Mariner, 1849, 1850; Mary, 1849; Passenger Lists Mary Shepherd, 1873; Mataura, 1863; Melbourne, The Library holds the two-volume listing of 1861; Merope, 1871; Mooltan, 1849; Nelson, 1888; Otago/Southland Assisted Passengers 1872–1888, who Nourmahal, 1858; Oriental, 1840; Oruba, 1901; arrived at Port Chalmers or Bluff. The lists give Palmerston, 1872; Parsee, 1874; Pekin, 1849; Peter occupations, ages, countries of origin and the year of Denny, 1873; Philip Laing, 1848; Pladda, 1861; arrival. Other composite lists are Passenger Lists to Poictiers, 1850; Queen of the North, 1862; Rajah, Otago 1869–1875, from the Otago Provincial 1853; Resolute, 1865; Robert Henderson, 1858, 1860, Gazettes; Paying Passengers to Otago 1871–80 by 1861, 1870; Royal Stuart, 1861; Schleswig Bride, P. Henderson and Co. Ships; and an Inventory of 1868; Scimitar, 1874; Sevilla, 1859, 1862; Shun Lee, Holdings, 1839–1888, at the Auckland Public Library 1871; Silistria, 1860, 1862; Sir Charles Forbes, 1842; (1968). -
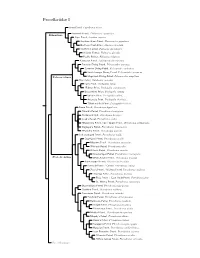
Procellariidae Species Tree
Procellariidae I Snow Petrel, Pagodroma nivea Antarctic Petrel, Thalassoica antarctica Fulmarinae Cape Petrel, Daption capense Southern Giant-Petrel, Macronectes giganteus Northern Giant-Petrel, Macronectes halli Southern Fulmar, Fulmarus glacialoides Atlantic Fulmar, Fulmarus glacialis Pacific Fulmar, Fulmarus rodgersii Kerguelen Petrel, Aphrodroma brevirostris Peruvian Diving-Petrel, Pelecanoides garnotii Common Diving-Petrel, Pelecanoides urinatrix South Georgia Diving-Petrel, Pelecanoides georgicus Pelecanoidinae Magellanic Diving-Petrel, Pelecanoides magellani Blue Petrel, Halobaena caerulea Fairy Prion, Pachyptila turtur ?Fulmar Prion, Pachyptila crassirostris Broad-billed Prion, Pachyptila vittata Salvin’s Prion, Pachyptila salvini Antarctic Prion, Pachyptila desolata ?Slender-billed Prion, Pachyptila belcheri Bonin Petrel, Pterodroma hypoleuca ?Gould’s Petrel, Pterodroma leucoptera ?Collared Petrel, Pterodroma brevipes Cook’s Petrel, Pterodroma cookii ?Masatierra Petrel / De Filippi’s Petrel, Pterodroma defilippiana Stejneger’s Petrel, Pterodroma longirostris ?Pycroft’s Petrel, Pterodroma pycrofti Soft-plumaged Petrel, Pterodroma mollis Gray-faced Petrel, Pterodroma gouldi Magenta Petrel, Pterodroma magentae ?Phoenix Petrel, Pterodroma alba Atlantic Petrel, Pterodroma incerta Great-winged Petrel, Pterodroma macroptera Pterodrominae White-headed Petrel, Pterodroma lessonii Black-capped Petrel, Pterodroma hasitata Bermuda Petrel / Cahow, Pterodroma cahow Zino’s Petrel / Madeira Petrel, Pterodroma madeira Desertas Petrel, Pterodroma