Bilateral Muscular Slips Between Superior and Inferior Rectus Muscles: Case Report with Discussion on Classification of Accessory Rectus Muscles Within the Orbit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -

CHQ-GDL-01074 Acute Management of Open Globe Injuries
Acute management of Open Globe Injuries Document ID CHQ-GDL-01074 Version no. 2.0 Approval date 14/05/2020 Executive sponsor Executive Director Medical Services Effective date 14/05/2020 Author/custodian Director Infection Management and Prevention service, Review date 14/05/2022 Immunology and Rheumatology Supersedes 1.0 Applicable to All Children’s Health Queensland (CHQ) staff Authorisation Executive Director Clinical Services (QCH) Purpose This evidence-based guideline provides clinical practice advice for clinicians for the acute management of children with open globe injuries. A paediatric ophthalmology team must be actively involved in the management of all patients presenting with this condition. Scope This guideline applies to all Children’s Health Queensland (CHQ) Staff treating a child presenting for the management of open globe injury. Related documents • CHQ-GDL-01202 CHQ Paediatric Antibiocard: Empirical Antibiotic Guidelines • CHQ-PROC-01035 Antimicrobial Restrictions • CHQ Antimicrobial Restriction list • CHQ-GDL-01023 Tetanus Prophylaxis in Wound Management CHQ-GDL-01074- Acute management of Open Globe Injuries - 1 - Guideline Introduction Ocular trauma is an important cause of eye morbidity and is a leading cause of non-congenital mono-ocular blindness among children.1 A quarter of a million children present each year with serious ocular trauma. The vast majority of these are preventable.2 Open globe injuries are injuries where the cornea and/or sclera are breached and there is a full-thickness wound of the eye wall.3 It can be further delineated into globe rupture from blunt trauma and lacerations from sharp objects. When a large blunt object impacts onto the eye, there is an instant increase in intraocular pressure and the eye wall yields at its weakest point leading to tissue prolapse.4 Open globe lacerations are caused by sharp objects or projectiles and subdivided into either penetrating or perforating injuries. -

Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts After Long-Duration Space Flight
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln NASA Publications National Aeronautics and Space Administration 10-2011 Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight Thomas H. Mader Alaska Native Medical Center, [email protected] C. Robert Gibson Coastal Eye Associates Anastas F. Pass University of Houston Larry A. Kramer University of Texas Health Science Center Andrew G. Lee The Methodist Hospital See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/nasapub Part of the Physical Sciences and Mathematics Commons Mader, Thomas H.; Gibson, C. Robert; Pass, Anastas F.; Kramer, Larry A.; Lee, Andrew G.; Fogarty, Jennifer; Tarver, William J.; Dervay, Joseph P.; Hamilton, Douglas R.; Sargsyan, Ashot; Phillips, John L.; Tran, Duc; Lipsky, William; Choi, Jung; Stern, Claudia; Kuyumjian, Raffi; andolk, P James D., "Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight" (2011). NASA Publications. 69. https://digitalcommons.unl.edu/nasapub/69 This Article is brought to you for free and open access by the National Aeronautics and Space Administration at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in NASA Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Thomas H. Mader, C. Robert Gibson, Anastas F. Pass, Larry A. -

Extraocular Muscles Orbital Muscles
EXTRAOCULAR MUSCLES ORBITAL MUSCLES INTRA- EXTRA- OCULAR OCULAR CILIARY MUSCLES INVOLUNTARY VOLUNTARY 1.Superior tarsal muscle. 1.Levator Palpebrae Superioris 2.Inferior tarsal muscle 2.Superior rectus 3.Inferior rectus 4.Medial rectus 5.Lateral rectus 6.Superior oblique 7.Inferior oblique LEVATOR PALPEBRAE SUPERIORIOS Origin- Inferior surface of lesser wing of sphenoid. Insertion- Upper lamina (Voluntary) - Anterior surface of superior tarsus & skin of upper eyelid. Middle lamina (Involuntary) - Superior margin of superior tarsus. (Superior Tarsus Muscle / Muller muscle) Lower lamina (Involuntary) - Superior conjunctival fornix Nerve Supply :- Voluntary part – Oculomotor Nerve Involuntary part – Sympathetic ACTION :- Elevation of upper eye lid C/S :- Drooping of upper eyelid. Congenital ptosis due to localized myogenic dysgenesis Complete ptosis - Injury to occulomotor nerve. Partial ptosis - disruption of postganglionic sympathetic fibres from superior cervical sympathetic ganglion. Extra ocular Muscles : Origin Levator palpebrae superioris Superior Oblique Superior Rectus Lateral Rectus Medial Rectus Inferior Oblique Inferior Rectus RECTUS MUSCLES : ORIGIN • Arises from a common tendinous ring knows as ANNULUS OF ZINN • Common ring of connective tissue • Anterior to optic foramen • Forms a muscle cone Clinical Significance Retrobulbar neuritis ○ Origin of SUPERIOR AND MEDIAL RECTUS are closely attached to the dural sheath of the optic nerve, which leads to pain during upward & inward movements of the globe. Thyroid orbitopathy ○ Medial & Inf.rectus thicken. especially near the orbital apex - compression of the optic nerve as it enters the optic canal adjacent to the body of the sphenoid bone. Ophthalmoplegia ○ Proptosis occur due to muscle laxity. Medial Rectus Superior Rectus Origin :- Superior limb of the tendonous ring, and optic nerve sheath. -
Periorbital Sinuses the Periorbital Sinuses Have a Close Anatomical Relationship with the Orbits (Fig 1-8)
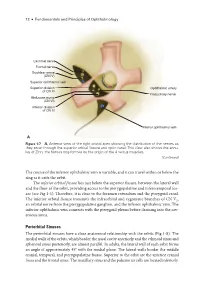
12 ● Fundamentals and Principles of Ophthalmology Lacrimal nerve Frontal nerve Trochlear nerve (CN IV) Superior ophthalmic vein Superior division Ophthalmic artery of CN III Nasociliary nerve Abducens nerve (CN VI) Inferior division of CN III Inferior ophthalmic vein A Figure 1-7 A, Anterior view of the right orbital apex showing the distribution of the nerves as they enter through the superior orbital fissure and optic canal. This view also shows the annu- lus of Zinn, the fibrous ring formed by the origin of the 4 rectus muscles. (Continued) The course of the inferior ophthalmic vein is variable, and it can travel within or below the ring as it exits the orbit. The inferior orbital fissure lies just below the superior fissure, between the lateral wall and the floor of the orbit, providing access to the pterygopalatine and inferotemporal fos- sae (see Fig 1-1). Therefore, it is close to the foramen rotundum and the pterygoid canal. The inferior orbital fissure transmits the infraorbital and zygomatic branches of CN V2, an orbital nerve from the pterygopalatine ganglion, and the inferior ophthalmic vein. The inferior ophthalmic vein connects with the pterygoid plexus before draining into the cav- ernous sinus. Periorbital Sinuses The periorbital sinuses have a close anatomical relationship with the orbits (Fig 1-8). The medial walls of the orbits, which border the nasal cavity anteriorly and the ethmoid sinus and sphenoid sinus posteriorly, are almost parallel. In adults, the lateral wall of each orbit forms an angle of approximately 45° with the medial plane. The lateral walls border the middle cranial, temporal, and pterygopalatine fossae. -

Trauma Suturing Techniques 7 Marian S
Chapter 7 Trauma Suturing Techniques 7 Marian S. Macsai and Bruno Machado Fontes Key Points 7.1 Introduction • Assess the presence of life-threatening inju- ries. Ocular trauma is an important cause of unilateral vi- • Vision at the time of presentation and the sion loss worldwide, especially in young people, and presence or absence of aff erent pupillary de- surgical repair is almost always challenging [1–7]. A fect are important prognostic factors in the patient with an eye injury may need immediate inter- Ocular Trauma Classifi cation System [1]. vention, and all ophthalmologists who cover emergen- • Surgical goals include: cy patients must have the knowledge and skills to deal – Watertight wound closure with diffi cult and complex surgeries, as these initial ac- – Restoration of normal anatomic relation- tions and interventions may be determinants for the ships fi nal visual prognosis [7–15]. One must keep in the – Restoration of optimal visual function mind that the result of the fi rst surgery will determine – Prevention of possible future complica- the need for future reconstruction. tions Th e epidemiology of ocular trauma varies according • Surgical indications: to the region studied. In the World Trade Center disas- – Any perforating injury ter, ocular trauma was found to be the second most – Any wound with tissue loss common type of injury among survivors [16]. Th e – Any clinical suspicion of globe rupture re- most common causes of eye injuries include automo- quires exploration and possible repair tive, domestic, and occupational accidents, together • Instrumentation: with violence. Risk factors most commonly described – Complete ophthalmic microsurgical tray for eye injuries are male gender (approximately 80% of – Phacoemulsifi cation, vitrectomy and irri- open-globe injuries), race (Hispanics and African- gation and aspiration machines Americans have higher risk), professional activity (e. -
Globe Rupture and Protrusion of Intraocular Contents from Fall in Elderly Patient
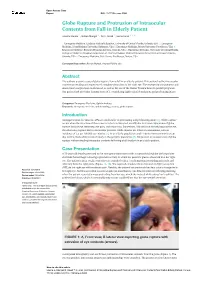
Open Access Case Report DOI: 10.7759/cureus.5988 Globe Rupture and Protrusion of Intraocular Contents from Fall in Elderly Patient Andrew Hanna 1 , Rohan Mangal 2 , Tej G. Stead 3 , Latha Ganti 4, 5, 6 1. Emergency Medicine, Graduate Medical Education, University of Central Florida, Orlando, USA 2. Emergency Medicine, Johns Hopkins University, Baltimore, USA 3. Emergency Medicine, Brown University, Providence, USA 4. Emergency Medicine, Envision Physician Services, Orlando, USA 5. Emergency Medicine, University of Central Florida College of Medicine / Hospital Corporation of America Graduate Medical Education Consortium of Greater Orlando, Orlando, USA 6. Emergency Medicine, Polk County Fire Rescue, Bartow, USA Corresponding author: Rohan Mangal, [email protected] Abstract The authors present a case of globe rupture from a fall in an elderly patient. This patient had her intraocular contents protruding and experienced complete vision loss in her right eye. The emergency management and downstream surgical care is discussed, as well as the use of the Ocular Trauma Score to predict prognosis. Our patient had an Ocular Trauma Score of 1, considering right retinal detachment and perforating injury. Categories: Emergency Medicine, Ophthalmology Keywords: emergency medicine, ophthalmology, trauma, globe rupture Introduction Amongst serious eye injuries, 40% are attributable to penetrating and perforating injury [1]. Globe rupture occurs when the structure of the cornea or sclera is disrupted, usually due to trauma. Symptoms of globe rupture include eye deformity, eye pain, and vision loss. Sometimes, if blunt force directly impacts the eye, the sclera may rupture due to intraocular pressure. Globe injuries are relatively uncommon, with an incidence of 3.5 per 100,000 eye injuries [2]. -

98796-Anatomy of the Orbit
Anatomy of the orbit Prof. Pia C Sundgren MD, PhD Department of Diagnostic Radiology, Clinical Sciences, Lund University, Sweden Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lay-out • brief overview of the basic anatomy of the orbit and its structures • the orbit is a complicated structure due to its embryological composition • high number of entities, and diseases due to its composition of ectoderm, surface ectoderm and mesoderm Recommend you to read for more details Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 3 x 3 Imaging technique 3 layers: - neuroectoderm (retina, iris, optic nerve) - surface ectoderm (lens) • CT and / or MR - mesoderm (vascular structures, sclera, choroid) •IOM plane 3 spaces: - pre-septal •thin slices extraconal - post-septal • axial and coronal projections intraconal • CT: soft tissue and bone windows 3 motor nerves: - occulomotor (III) • MR: T1 pre and post, T2, STIR, fat suppression, DWI (?) - trochlear (IV) - abducens (VI) Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Superior orbital fissure • cranial nerves (CN) III, IV, and VI • lacrimal nerve • frontal nerve • nasociliary nerve • orbital branch of middle meningeal artery • recurrent branch of lacrimal artery • superior orbital vein • superior ophthalmic vein Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. -

Evolution of Nervous Systems and Brains 2
Evolution of Nervous Systems and Brains 2 Gerhard Roth and Ursula Dicke The modern theory of biological evolution, as estab- drift”) is incomplete; they point to a number of other lished by Charles Darwin and Alfred Russel Wallace and perhaps equally important mechanisms such as in the middle of the nineteenth century, is based on (i) neutral gene evolution without natural selection, three interrelated facts: (i) phylogeny – the common (ii) mass extinctions wiping out up to 90 % of existing history of organisms on earth stretching back over 3.5 species (such as the Cambrian, Devonian, Permian, and billion years, (ii) evolution in a narrow sense – Cretaceous-Tertiary mass extinctions) and (iii) genetic modi fi cations of organisms during phylogeny and and epigenetic-developmental (“ evo - devo ”) self-canal- underlying mechanisms, and (iii) speciation – the ization of evolutionary processes [ 2 ] . It remains uncer- process by which new species arise during phylogeny. tain as to which of these possible processes principally Regarding the phylogeny, it is now commonly accepted drive the evolution of nervous systems and brains. that all organisms on Earth are derived from a com- mon ancestor or an ancestral gene pool, while contro- versies have remained since the time of Darwin and 2.1 Reconstruction of the Evolution Wallace about the major mechanisms underlying the of Nervous Systems and Brains observed modi fi cations during phylogeny (cf . [1 ] ). The prevalent view of neodarwinism (or better In most cases, the reconstruction of the evolution of “new” or “modern evolutionary synthesis”) is charac- nervous systems and brains cannot be based on fossil- terized by the assumption that evolutionary changes ized material, since their soft tissues decompose, but are caused by a combination of two major processes, has to make use of the distribution of neural traits in (i) heritable variation of individual genomes within a extant species. -

A Pictorial Anatomy of the Human Eye/Anophthalmic Socket: a Review for Ocularists
A Pictorial Anatomy of the Human Eye/Anophthalmic Socket: A Review for Ocularists ABSTRACT: Knowledge of human eye anatomy is obviously impor- tant to ocularists. This paper describes, with pictorial emphasis, the anatomy of the eye that ocularists generally encounter: the anophthalmic eye/socket. The author continues the discussion from a previous article: Anatomy of the Anterior Eye for Ocularists, published in 2004 in the Journal of Ophthalmic Prosthetics.1 Michael O. Hughes INTRODUCTION AND RATIONALE B.C.O. Artificial Eye Clinic of Washington, D.C. Understanding the basic anatomy of the human eye is a requirement for all Vienna, Virginia health care providers, but it is even more significant to eye care practition- ers, including ocularists. The type of eye anatomy that ocularists know, how- ever, is more abstract, as the anatomy has been altered from its natural form. Although the companion eye in monocular patients is usually within the normal range of aesthetics and function, the affected side may be distorted. While ocularists rarely work on actual eyeballs (except to cover microph- thalmic and blind, phthisical eyes using scleral cover shells), this knowledge can assist the ocularist in obtaining a naturally appearing prosthesis, and it will be of greater benefit to the patient. An easier exchange among ocularists, surgeons, and patients will result from this knowledge.1, 2, 3 RELATIONSHIPS IN THE NORMAL EYE AND ORBIT The opening between the eyelids is called the palpebral fissure. In the nor- mal eye, characteristic relationships should be recognized by the ocularist to understand the elements to be evaluated in the fellow eye. -

Anatomy of the Periorbital Region Review Article Anatomia Da Região Periorbital
RevSurgicalV5N3Inglês_RevistaSurgical&CosmeticDermatol 21/01/14 17:54 Página 245 245 Anatomy of the periorbital region Review article Anatomia da região periorbital Authors: Eliandre Costa Palermo1 ABSTRACT A careful study of the anatomy of the orbit is very important for dermatologists, even for those who do not perform major surgical procedures. This is due to the high complexity of the structures involved in the dermatological procedures performed in this region. A 1 Dermatologist Physician, Lato sensu post- detailed knowledge of facial anatomy is what differentiates a qualified professional— graduate diploma in Dermatologic Surgery from the Faculdade de Medician whether in performing minimally invasive procedures (such as botulinum toxin and der- do ABC - Santo André (SP), Brazil mal fillings) or in conducting excisions of skin lesions—thereby avoiding complications and ensuring the best results, both aesthetically and correctively. The present review article focuses on the anatomy of the orbit and palpebral region and on the important structures related to the execution of dermatological procedures. Keywords: eyelids; anatomy; skin. RESU MO Um estudo cuidadoso da anatomia da órbita é muito importante para os dermatologistas, mesmo para os que não realizam grandes procedimentos cirúrgicos, devido à elevada complexidade de estruturas envolvidas nos procedimentos dermatológicos realizados nesta região. O conhecimento detalhado da anatomia facial é o que diferencia o profissional qualificado, seja na realização de procedimentos mini- mamente invasivos, como toxina botulínica e preenchimentos, seja nas exéreses de lesões dermatoló- Correspondence: Dr. Eliandre Costa Palermo gicas, evitando complicações e assegurando os melhores resultados, tanto estéticos quanto corretivos. Av. São Gualter, 615 Trataremos neste artigo da revisão da anatomia da região órbito-palpebral e das estruturas importan- Cep: 05455 000 Alto de Pinheiros—São tes correlacionadas à realização dos procedimentos dermatológicos. -

Traumatic Keratoplasty Rupture Resulting from Continuous Positive Airway Pressure Mask
CASE REPORT Traumatic Keratoplasty Rupture Resulting From Continuous Positive Airway Pressure Mask Miltiadis Fiorentzis, MD, Berthold Seitz, MD, and Arne Viestenz, MD We report an unusual case of traumatic wound Purpose: To report a rare case of traumatic wound dehiscence dehiscence due to a dislocation of a continuous positive caused by the use of a continuous positive airway pressure (CPAP) airway pressure (CPAP) mask during sleep after PKP. To the mask in a patient with chronic obstructive pulmonary disease best of our knowledge, this is the first report of this unusual (COPD) after penetrating keratoplasty (PKP). graft separation cause. Methods: Observational case report. CASE REPORT Case report: A 55-year-old man who was treated with uncompli- cated PKP due to pellucid marginal corneal degeneration in the right A 55-year-old man underwent an excimer laser-assisted PKP eye 9 months earlier presented to the emergency department after because of pellucid marginal corneal degeneration in the right eye. The postoperative course was uncomplicated (Fig. 1A). His medical a globe rupture caused by dislocation of his CPAP mask during history revealed chronic obstructive pulmonary disease (COPD), sleep. The best-corrected visual acuity (BCVA) was light perception treated with a CPAP mask. Three months after surgery and under in the right eye. The corneal graft was dehisced from 12 over 3 to 6 treatment with topical steroids 3 times a day, the BCVA was 20/32, o’clock (180 degrees) with interruption of the double running the corneal astigmatism approximately 1.8 diopters, and the central corneal sutures and nasal iris as well as vitreous incarceration.