(TRP) Cation Channels Could Explain Smell, Taste, And/Or Chemesthesis Disorders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oral Thermosensing by Murine Trigeminal Neurons: Modulation by Capsaicin, Menthol, and Mustard Oil
bioRxiv preprint doi: https://doi.org/10.1101/486480; this version posted December 4, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Oral thermosensing by murine trigeminal neurons: modulation by capsaicin, menthol, and mustard oil Sara C.M. Leijon1, Amanda F. Neves1, Sidney A. Simon2, Nirupa Chaudhari1,3, Stephen D. Roper1,3 1) Department of Physiology & Biophysics, Miller School of Medicine, University of Miami, Miami, FL, USA 2) Department of Neurobiology, Duke University, Durham, NC, USA 3) Department of Otolaryngology, Miller School of Medicine, University of Miami, and Program in Neuroscience, University of Miami, Miami, FL, USA Running title: Trigeminal orosensory responses in the mouse Key words: Trigeminal ganglion, sensory neurons, calcium imaging, thermosensation, chemesthesis Key points summary Orosensory thermal trigeminal afferent neurons respond to cool, warm, and nociceptive hot temperatures with the majority activated in the cool range. Many of these thermosensitive trigeminal orosensory afferent neurons also respond to capsaicin, menthol and/or mustard oil (allyl isothiocyanate, AITC) at concentrations found in foods and spices. There is significant but incomplete overlap between afferent trigeminal neurons that respond to heat and to the above chemesthetic compounds. Capsaicin sensitizes warm trigeminal thermoreceptors and orosensory nociceptors; menthol attenuates cool thermoresponses. 1 bioRxiv preprint doi: https://doi.org/10.1101/486480; this version posted December 4, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract When consumed with foods, mint, mustard and chili peppers generate pronounced oral thermosensations. -

More Than Smell. COVID-19 Is Associated with Severe Impairment
medRxiv preprint doi: https://doi.org/10.1101/2020.05.04.20090902.this version posted May 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC 4.0 International license . 1 More than smell – COVID-19 is associated with severe impairment of smell, taste, and chemesthesis By-line Authors: Valentina Parma1*, Kathrin Ohla2*, Maria G. Veldhuizen3*, Masha Y Niv4, Christine E Kelly5, Alyssa J. Bakke6, Keiland W. Cooper7, Cédric Bouysset8, Nicola Pirastu9, Michele Dibattista10, RishemJit Kaur11, Marco Tullio Liuzza12, Marta Y. Pepino13, Veronika Schöpf14, Veronica Pereda- Loth15, Shannon B Olsson16, Richard C Gerkin17, Paloma Rohlfs Domínguez18, Javier Albayay19, Michael C. Farruggia20, Surabhi Bhutani21, Alexander W. FJaeldstad22, Ritesh Kumar23, Anna Menini24, Moustafa Bensafi25, Mari Sandell26, Iordanis Konstantinidis27, Antonella Di Pizio28, Federica Genovese29, Lina Öztürk30, Thierry Thomas-Danguin31, Johannes Frasnelli32, Sanne Boesveldt33, Özlem Saatci34, Luis R. Saraiva35, Cailu Lin29, Jérôme Golebiowski36, Liang-Dar Hwang37, Mehmet Hakan Ozdener29, Maria Dolors Guàrdia38, Christophe Laudamiel39, Marina Ritchie40, Jan Havlícek41, Denis Pierron42, Eugeni Roura43, Marta Navarro43, Alissa A. Nolden44, Juyun Lim45, KL Whitcroft46, Lauren R Colquitt29, Camille Ferdenzi47, Evelyn V Brindha48, Aytug Altundag49, Alberto Macchi50, Alexia Nunez-Parra51, Zara M. Patel52, Sébastien Fiorucci36, Carl M Philpott53, Barry C. Smith54, Johan N. Lundström55, Carla Mucignat56, Jane K. Parker57, Mirjam van den Brink58, Michael Schmuker59, Florian Ph.S Fischmeister60, Thomas Heinbockel61, Vonnie D.C. Shields62, Farhoud FaraJi63, Enrique Santamaría64, William E.A. Fredborg65, Gabriella Morini66, Jonas K. Olofsson65, Maryam Jalessi67, Noam Karni68, Anna D’Errico69, Rafieh Alizadeh70, Robert Pellegrino71, Pablo Meyer72, Caroline Huart73, Ben Chen74, Graciela M. -

Transient Receptor Potential Channel Promiscuity Frustrates Constellation
the sole sensor responsible for noxious cold responses in M+A+ LETTER neurons (1). However, TRPA1 is also activated by cooling and underlies at least part of the noxious cold responsiveness of Transient receptor potential channel AITC-sensitive neurons (3). Third, the authors used nicardipine 2+ promiscuity frustrates to selectively inhibit CaV1-type voltage-gated Ca channels (1). However, several dihydropyridines, including nicardipine, also constellation pharmacology act as TRPA1 agonists (4). These considerations led us to propose an alternative Sensory neurons from the trigeminal and dorsal root ganglia molecular interpretation of the difference between M+A− (DRG) have nerve endings in the skin and mucosa, where they and M+A+ neurons, which is in much better agreement with detect environmental stimuli and convey this information to published work. In accord with the authors, we conclude that the central nervous system. Several members of the transient M+A− neurons express TRPM8 but lack expression of receptor potential (TRP) superfamily of ion channels act as TRPA1. In contrast to the authors, we propose that M+A+ prime molecular sensors for thermal and chemical stimuli in neurons express TRPA1 as the prime cold and menthol these sensory neurons. However, it is incompletely understood sensor (2, 3). This interpretation is consistent with published how TRP channel expression and modulation affect the stimulus observations that menthol responses in M+A− but not in sensitivities of distinct neuronal subtypes. M+A+ neurons are inhibited by TRPM8 antagonists (5) In a recent article, Teichert et al. (1) described a “constellation and that TRPA1-mediated responses to cold in neurons are pharmacology approach” to identify and characterize subtypes characterized by a higher (colder) threshold (3). -

Ankrd9 Is a Metabolically-Controlled Regulator of Impdh2 Abundance and Macro-Assembly
ANKRD9 IS A METABOLICALLY-CONTROLLED REGULATOR OF IMPDH2 ABUNDANCE AND MACRO-ASSEMBLY by Dawn Hayward A dissertation submitted to The Johns Hopkins University in conformity with the requirements of the degree of Doctor of Philosophy Baltimore, Maryland April 2019 ABSTRACT Members of a large family of Ankyrin Repeat Domains proteins (ANKRD) regulate numerous cellular processes by binding and changing properties of specific protein targets. We show that interactions with a target protein and the functional outcomes can be markedly altered by cells’ metabolic state. ANKRD9 facilitates degradation of inosine monophosphate dehydrogenase 2 (IMPDH2), the rate-limiting enzyme in GTP biosynthesis. Under basal conditions ANKRD9 is largely segregated from the cytosolic IMPDH2 by binding to vesicles. Upon nutrient limitation, ANKRD9 loses association with vesicles and assembles with IMPDH2 into rod-like structures, in which IMPDH2 is stable. Inhibition of IMPDH2 with Ribavirin favors ANKRD9 binding to rods. The IMPDH2/ANKRD9 assembly is reversed by guanosine, which restores association of ANKRD9 with vesicles. The conserved Cys109Cys110 motif in ANKRD9 is required for the vesicles-to-rods transition as well as binding and regulation of IMPDH2. ANKRD9 knockdown increases IMPDH2 levels and prevents formation of IMPDH2 rods upon nutrient limitation. Thus, the status of guanosine pools affects the mode of ANKRD9 action towards IMPDH2. Advisor: Dr. Svetlana Lutsenko, Department of Physiology, Johns Hopkins University School of Medicine Second reader: -

Chapter Four – TRPA1 Channels: Chemical and Temperature Sensitivity
CHAPTER FOUR TRPA1 Channels: Chemical and Temperature Sensitivity Willem J. Laursen1,2, Sviatoslav N. Bagriantsev1,* and Elena O. Gracheva1,2,* 1Department of Cellular and Molecular Physiology, Yale University School of Medicine, New Haven, CT, USA 2Program in Cellular Neuroscience, Neurodegeneration and Repair, Yale University School of Medicine, New Haven, CT, USA *Corresponding author: E-mail: [email protected], [email protected] Contents 1. Introduction 90 2. Activation and Regulation of TRPA1 by Chemical Compounds 91 2.1 Chemical activation of TRPA1 by covalent modification 91 2.2 Noncovalent activation of TRPA1 97 2.3 Receptor-operated activation of TRPA1 99 3. Temperature Sensitivity of TRPA1 101 3.1 TRPA1 in mammals 101 3.2 TRPA1 in insects and worms 103 3.3 TRPA1 in fish, birds, reptiles, and amphibians 103 3.4 TRPA1: Molecular mechanism of temperature sensitivity 104 Acknowledgments 107 References 107 Abstract Transient receptor potential ankyrin 1 (TRPA1) is a polymodal excitatory ion channel found in sensory neurons of different organisms, ranging from worms to humans. Since its discovery as an uncharacterized transmembrane protein in human fibroblasts, TRPA1 has become one of the most intensively studied ion channels. Its function has been linked to regulation of heat and cold perception, mechanosensitivity, hearing, inflam- mation, pain, circadian rhythms, chemoreception, and other processes. Some of these proposed functions remain controversial, while others have gathered considerable experimental support. A truly polymodal ion channel, TRPA1 is activated by various stimuli, including electrophilic chemicals, oxygen, temperature, and mechanical force, yet the molecular mechanism of TRPA1 gating remains obscure. In this review, we discuss recent advances in the understanding of TRPA1 physiology, pharmacology, and molecular function. -

Trpc, Trpv and Vascular Disease | Encyclopedia
TRPC, TRPV and Vascular Disease Subjects: Biochemistry Submitted by: Tarik Smani Hajami Definition Ion channels play an important role in vascular function and pathology. In this review we gave an overview of recent findings and discussed the role of TRPC and TRPV channels as major regulators of cellular remodeling and consequent vascular disorders. Here, we focused on their implication in 4 relevant vascular diseases: systemic and pulmonary artery hypertension, atherosclerosis and restenosis. Transient receptor potentials (TRPs) are non-selective cation channels that are widely expressed in vascular beds. They contribute to the Ca2+ influx evoked by a wide spectrum of chemical and physical stimuli, both in endothelial and vascular smooth muscle cells. Within the superfamily of TRP channels, different isoforms of TRPC (canonical) and TRPV (vanilloid) have emerged as important regulators of vascular tone and blood flow pressure. Additionally, several lines of evidence derived from animal models, and even from human subjects, highlighted the role of TRPC and TRPV in vascular remodeling and disease. Dysregulation in the function and/or expression of TRPC and TRPV isoforms likely regulates vascular smooth muscle cells switching from a contractile to a synthetic phenotype. This process contributes to the development and progression of vascular disorders, such as systemic and pulmonary arterial hypertension, atherosclerosis and restenosis. 1. Introduction Blood vessels are composed essentially of two interacting cell types: endothelial cells (ECs) from the tunica intima lining of the vessel wall and vascular smooth muscle cells (VSMCs) from tunica media of the vascular tube. Blood vessels are a complex network, and they differ according to the tissue to which they belong, having diverse cell expressions, structures and functions [1][2][3]. -

Repeat Proteins Challenge the Concept of Structural Domains
844 Biochemical Society Transactions (2015) Volume 43, part 5 Repeat proteins challenge the concept of structural domains Rocıo´ Espada*1, R. Gonzalo Parra*1, Manfred J. Sippl†, Thierry Mora‡, Aleksandra M. Walczak§ and Diego U. Ferreiro*2 *Protein Physiology Lab, Dep de Qu´ımica Biologica, ´ Facultad de Ciencias Exactas y Naturales, UBA-CONICET-IQUIBICEN, Buenos Aires, C1430EGA, Argentina †Center of Applied Molecular Engineering, Division of Bioinformatics, Department of Molecular Biology, University of Salzburg, 5020 Salzburg, Austria ‡Laboratoire de physique statistique, CNRS, UPMC and Ecole normale superieure, ´ 24 rue Lhomond, 75005 Paris, France §Laboratoire de physique theorique, ´ CNRS, UPMC and Ecole normale superieure, ´ 24 rue Lhomond, 75005 Paris, France Abstract Structural domains are believed to be modules within proteins that can fold and function independently. Some proteins show tandem repetitions of apparent modular structure that do not fold independently, but rather co-operate in stabilizing structural forms that comprise several repeat-units. For many natural repeat-proteins, it has been shown that weak energetic links between repeats lead to the breakdown of co-operativity and the appearance of folding sub-domains within an apparently regular repeat array. The quasi-1D architecture of repeat-proteins is crucial in detailing how the local energetic balances can modulate the folding dynamics of these proteins, which can be related to the physiological behaviour of these ubiquitous biological systems. Introduction and between repeats, challenging the concept of structural It was early on noted that many natural proteins typically domain. collapse stretches of amino acid chains into compact units, defining structural domains [1]. These domains typically correlate with biological activities and many modern proteins can be described as composed by novel ‘domain arrange- Definition of the repeat-units ments’ [2]. -

A Structural Guide to Proteins of the NF-Kb Signaling Module
Downloaded from http://cshperspectives.cshlp.org/ on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press A Structural Guide to Proteins of the NF-kB Signaling Module Tom Huxford1 and Gourisankar Ghosh2 1Department of Chemistry and Biochemistry, San Diego State University, 5500 Campanile Drive, San Diego, California 92182-1030 2Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, California 92093-0375 Correspondence: [email protected] The prosurvival transcription factor NF-kB specifically binds promoter DNA to activate target gene expression. NF-kB is regulated through interactions with IkB inhibitor proteins. Active proteolysis of these IkB proteins is, in turn, under the control of the IkB kinase complex (IKK). Together, these three molecules form the NF-kB signaling module. Studies aimed at charac- terizing the molecular mechanisms of NF-kB, IkB, and IKK in terms of their three-dimen- sional structures have lead to a greater understanding of this vital transcription factor system. F-kB is a master transcription factor that from the perspective of their three-dimensional Nresponds to diverse cell stimuli by activat- structures. ing the expression of stress response genes. Multiple signals, including cytokines, growth factors, engagement of the T-cell receptor, and NF-kB bacterial and viral products, induce NF-kB Introduction to NF-kB transcriptional activity (Hayden and Ghosh 2008). A point of convergence for the myriad NF-kB was discovered in the laboratory of of NF-kB inducing signals is the IkB kinase David Baltimore as a nuclear activity with bind- complex (IKK). Active IKK in turn controls ing specificity toward a ten-base-pair DNA transcription factor NF-kB by regulating pro- sequence 50-GGGACTTTCC-30 present within teolysis of the IkB inhibitor protein (Fig. -
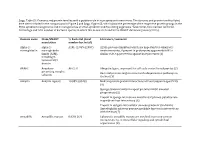
Supp. Table S2: Domains and Protein Families with a Putative Role in Host-Symbiont Interactions
Supp. Table S2: Domains and protein families with a putative role in host-symbiont interactions. The domains and protein families listed here were included in the comparisons in Figure 5 and Supp. Figure S5, which show the percentage of the respective protein groups in the Riftia symbiont metagenome and in metagenomes of other symbiotic and free-living organisms. % bacterial, total number bacterial: Percentage and total number of bacterial species in which this domain is found in the SMART database (January 2019). Domain name Pfam/SMART % bacterial (total Literature/comment annotation number bacterial) Alpha-2- alpha-2- A2M: 42.05% (2057) A2Ms: protease inhibitors which are important for eukaryotic macroglobulin macroglobulin innate immunity, if present in prokaryotes apparently fulfill a family (A2M), similar role, e.g. protection against host proteases (1) including N- terminal MG1 domain ANAPC Anaphase- APC2: 0 Ubiquitin ligase, important for cell cycle control in eukaryotes (2) promoting complex Bacterial proteins might interact with ubiquitination pathways in subunits the host (3) Ankyrin Ankyrin repeats 10.88% (8348) Mediate protein-protein interactions without sequence specificity (4) Sponge symbiont ankyrin-repeat proteins inhibit amoebal phagocytosis (5) Present in sponge microbiome metatranscriptomes, putative role in symbiont-host interactions (6) Present in obligate intracellular amoeba symbiont Candidatus Amoebophilus asiaticus genome, probable function in interactions with the host (7) Armadillo Armadillo repeats 0.83% (67) -

The Role of Pain Receptors in the Main Symptoms of Covid-19 and How Diet Can Be a Therapy
Internal Medicine: Open Access Review Article The Role of Pain Receptors in the Main Symptoms of Covid-19 and How Diet Can Be a Therapy Francesco Amato1*, Erminia Gilda Morrone2 1UOC Pain Therapy and CP Hospital, Cosenza, Italy; 2Association Center for Pain Therapy Studies, Cosenza, Italy ABSTRACT It is estimated that 80% of SARS-CoV-2 patients have olfactory disturbances and many also have dysgeusia or ageusia (an interruption or loss of taste, respectively) or changes in chemesthesis, the ability to perceive irritants by TRP receptors. Anosmia (loss of sense of smell) and dysgeusia been termed 'sentinel symptoms'. Anosmia and ageusia represent a real health risk and can also cause nutritional deficits'. Infection with SARS-CoV-2 in the oral cavity could cause changes in the production or quality of saliva, contributing to the symptoms of taste loss. Since the activation of TRPs by Reactive Oxygen Species (ROS) contributes to inflammation and pain, research is focusing on several biological mediators related to TRPs and oxidative radicals that could help the development of treatments for pain itself and some COVID related symptoms. Recent studies have found that Nuclear Factor Erythroid-Related Factor 2 (NRF2) is a transcription factor that regulates cellular defence against toxic and oxidative insults. Compounds that can activate or induce NRF2 include garlic H2S polysulphides, cinnaldehyde in cinnamon, polyphenols in green tea, curcumin, a polyphenolic compound found in curcuma, piperine, an alkaloid found in black pepper, and glucoraphanin found in broccoli. In addition, there is a substantial electrophilic interaction between NRF2, TRPA1 and TPV1 that results in their desensitisation. -

TRPM8 Channels and Dry Eye
UC Berkeley UC Berkeley Previously Published Works Title TRPM8 Channels and Dry Eye. Permalink https://escholarship.org/uc/item/2gz2d8s3 Journal Pharmaceuticals (Basel, Switzerland), 11(4) ISSN 1424-8247 Authors Yang, Jee Myung Wei, Edward T Kim, Seong Jin et al. Publication Date 2018-11-15 DOI 10.3390/ph11040125 Peer reviewed eScholarship.org Powered by the California Digital Library University of California pharmaceuticals Review TRPM8 Channels and Dry Eye Jee Myung Yang 1,2 , Edward T. Wei 3, Seong Jin Kim 4 and Kyung Chul Yoon 1,* 1 Department of Ophthalmology, Chonnam National University Medical School and Hospital, Gwangju 61469, Korea; [email protected] 2 Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology, Daejeon 34141, Korea 3 School of Public Health, University of California, Berkeley, CA 94720, USA; [email protected] 4 Department of Dermatology, Chonnam National University Medical School and Hospital, Gwangju 61469, Korea; [email protected] * Correspondence: [email protected] Received: 17 September 2018; Accepted: 12 November 2018; Published: 15 November 2018 Abstract: Transient receptor potential (TRP) channels transduce signals of chemical irritation and temperature change from the ocular surface to the brain. Dry eye disease (DED) is a multifactorial disorder wherein the eyes react to trivial stimuli with abnormal sensations, such as dryness, blurring, presence of foreign body, discomfort, irritation, and pain. There is increasing evidence of TRP channel dysfunction (i.e., TRPV1 and TRPM8) in DED pathophysiology. Here, we review some of this literature and discuss one strategy on how to manage DED using a TRPM8 agonist. -

Does Serotonin Deficiency Lead to Anosmia, Ageusia, Dysfunctional Chemesthesis and Increased Severity of Illness in COVID-19?
Does serotonin deficiency lead to anosmia, ageusia, dysfunctional chemesthesis and increased severity of illness in COVID-19? Amarnath Sen 40 Jadunath Sarbovouma Lane, Kolkata 700035, India, E-mail: [email protected] ABSTRACT Anosmia, ageusia and impaired chemesthetic sensations are quite common in coronavirus patients. Different mechanisms have been proposed to explain the anosmia and ageusia in COVID-19, though for reversible anosmia and ageusia, which are resolved quickly, the proposed mechanisms seem to be incomplete. In addition, the reason behind the impaired chemesthetic sensations in some coronavirus patients remains unknown. It is proposed that coronavirus patients suffer from depletion of tryptophan (an essential amino acid), as ACE2, a key element in the process of absorption of tryptophan from the food, is significantly reduced due to the attack of coronavirus, which use ACE2 as the receptor for its entry into the host cells. The depletion of tryptophan should lead to a deficit of serotonin (5-HT) in SARS-COV- 2 patients because tryptophan is the precursor in the synthesis of 5-HT. Such 5-HT deficiency can give rise to anosmia, ageusia and dysfunctional chemesthesis in COVID-19, given the fact that 5-HT is an important neuromodulator in the olfactory neurons and taste receptor cells and 5-HT also enhances the nociceptor activity of transient receptor potential channels (TRP channels) responsible for the chemesthetic sensations. In addition, 5-HT deficiency is expected to worsen silent hypoxemia and depress hypoxic pulmonary vasoconstriction (a protective reflex) leading to an increased severity of the disease and poor outcome. Melatonin, a potential adjuvant in the treatment of COVID-19, which can tone down cytokine storm, is produced from 5-HT and is expected to decrease due to the deficit of 5-HT in the coronavirus patients.