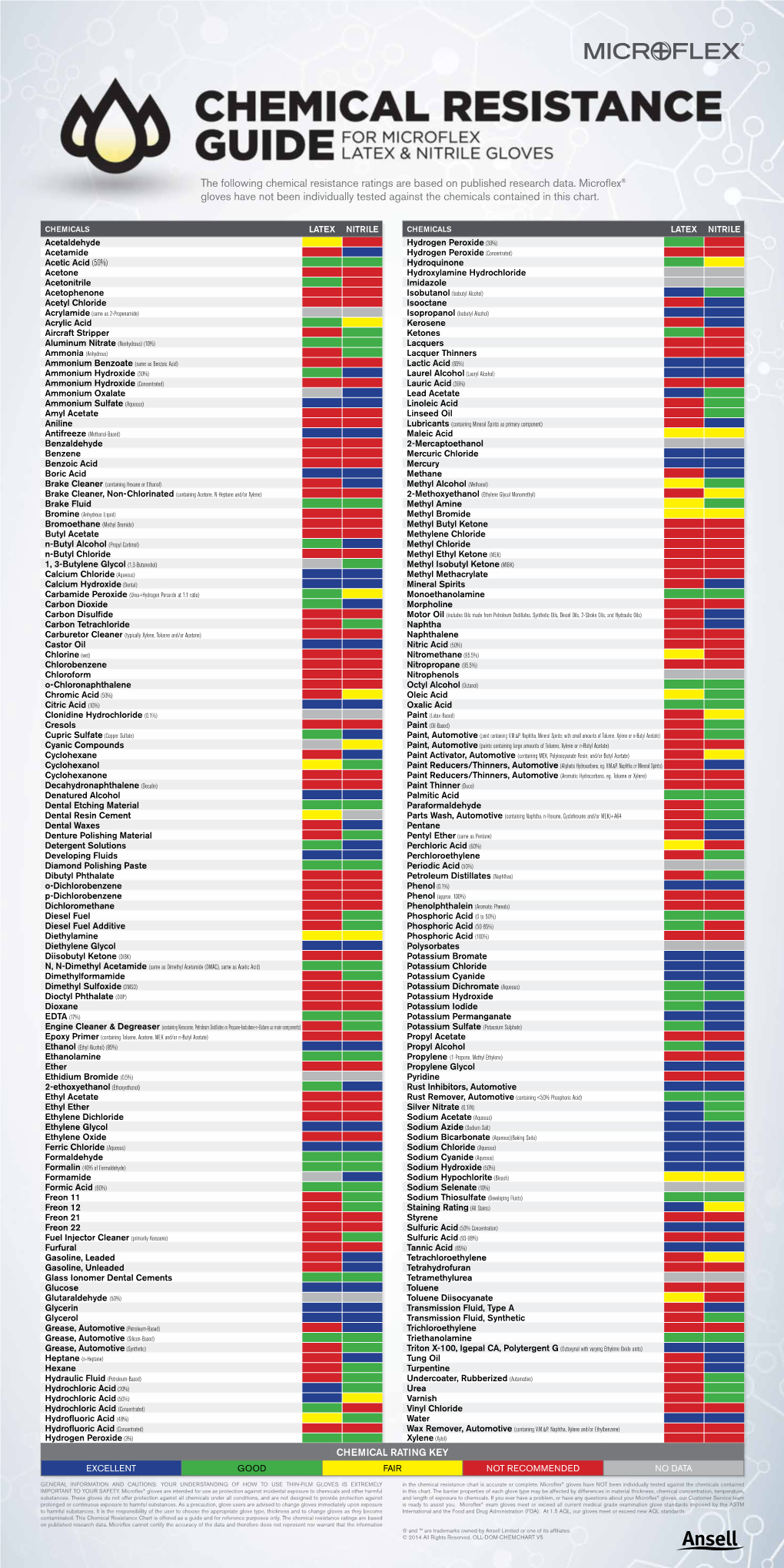
The Following Chemical Resistance Ratings Are Based on Published Research Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Agarose Gel Electrophoresis
Laboratory for Environmental Pathogen Research Department of Environmental Sciences University of Toledo Agarose gel electrophoresis Background information Agarose gel electrophoresis of DNA is used to determine the presence and distinguish the type of nucleic acids obtained after extraction and to analyze restriction digestion products. Desired DNA fragments can be physically isolated for various purposes such as sequencing, probe preparation, or for cloning fragments into other vectors. Both agarose and polyacrylamide gels are used for DNA analysis. Agarose gels are usually run to size larger fragments (greater than 200 bp) and polyacrylamide gels are run to size fragments less than 200 bp. Typically agarose gels are used for most purposes and polyacrylamide gels are used when small fragments, such as digests of 16S rRNA genes, are being distinguished. There are also specialty agaroses made by FMC (e.g., Metaphor) for separating small fragments. Regular agarose gels may range in concentration from 0.6 to 3.0%. Pouring gels at less or greater than these percentages presents handling problems (e.g., 0.4% agarose for genomic DNA partial digests requires a layer of supporting 0.8% gel). For normal samples make agarose gels at 0.7%. The chart below illustrates the optimal concentrations for fragment size separation. The values listed are approximate and can vary depending on the reference that is used. If you do not know your fragment sizes then the best approach is to start with a 0.7% gel and change subsequently if the desired separation is not achieved. Nucleic acids must be stained prior to visualization. Most laboratories use ethidium bromide but other stains (e.g., SYBR green, GelStar) are available. -

Gelred® and Gelgreen® Safety Report
Safety Report for GelRed® and GelGreen® A summary of mutagenicity and environmental safety test results from three independent laboratories for the nucleic acid gel stains GelRed® and GelGreen® www.biotium.com General Inquiries: [email protected] Technical Support: [email protected] Phone: 800-304-5357 Conclusion Overview GelRed® and GelGreen® are a new generation of nucleic acid gel stains. Ethidium bromide (EB) has been the stain of choice for nucleic acid gel They possess novel chemical features designed to minimize the chance for staining for decades. The dye is inexpensive, sufficiently sensitive and very the dyes to interact with nucleic acids in living cells. Test results confirm that stable. However, EB is also a known powerful mutagen. It poses a major the dyes do not penetrate latex gloves or cell membranes. health hazard to the user, and efforts in decontamination and waste disposal ultimately make the dye expensive to use. To overcome the toxicity problem In the AMES test, GelRed® and GelGreen® are noncytotoxic and of EB, scientists at Biotium developed GelRed® and GelGreen® nucleic acid nonmutagenic at concentrations well above the working concentrations gel stains as superior alternatives. Extensive tests demonstrate that both used in gel staining. The highest dye concentrations shown to be non-toxic dyes have significantly improved safety profiles over EB. and non-mutagenic in the Ames test for GelRed® and GelGreen® dyes are 18.5-times higher than the 1X working concentration used for gel casting, and 6-times higher than the 3X working concentration used for gel staining. This Dye Design Principle is in contrast to SYBR® Safe, which has been reported to show mutagenicity At the very beginning of GelRed® and GelGreen® development, we made a in several strains in the presence of S9 mix (1). -

Supplementary Data for Publication
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is © the Owner Societies 2016 Supplementary Data for Publication Synthesis of Eucalyptus/Tea Tree Oil Absorbed Biphasic Calcium phosphate-PVDF Polymer Nanocomposite Films: A Surface Active Antimicrobial System for Biomedical Application Biswajoy Bagchi1,δ, Somtirtha Banerjee1, Arpan Kool1, Pradip Thakur1,2, Suman Bhandary3, Nur Amin Hoque1 , Sukhen Das1+* 1Physics Department, Jadavpur University, Kolkata-700032, India. 2Department of Physics, Netaji Nagar College for Women, Kolkata-700092, India. 3Division of Molecular Medicine, Bose Institute, Kolkata-700054, India. +Present Address: Department of Physics, Indian Institute of Engineering Science and Technology, Shibpur, Howrah, West Bengal-711103, India. §Present Address: Fuel Cell and Battery Division, Central Glass and Ceramic Research Institute, Kolkata-700032, India. *Corresponding author’s email id: [email protected] Contact: +919433091337 Antimicrobial activity of EU and TTO treated films on E .coli and S. aureus by acridine orange/ethidium bromide (AO/EB) dual staining Live/dead cell characterization of EU/TTO film treated bacterial cultures was also done to visualize the viability under fluorescence microscope (). The treated culture suspensions after 12 and 24 hours of incubation were collected by centrifugation (5000 rpm, 20 mins). The cell pellets were resuspended in PBS. The staining solution was prepared by mixing equal parts of acridine orange (5mg/mL) and ethidium bromide (3mg/mL) in ethanol. 20μL of the staining solution is then mixed with 10μL of the resuspended solution and incubated for 15 minutes at 37°C. 10μL of this solution was then placed on a glass slide and covered with cover slip to observe under fluorescence microscope. -

Ethidium Bromide Alternatives Assessment August 2009 (Revised: August 2011)
MIT EHS Office Green Chemistry/Pollution Prevention Program Ethidium Bromide Alternatives Assessment August 2009 (revised: August 2011) Product Nucleic acids Visual Gel base and Sensitivity Stability or Types of visual Mutagenicity, Disposal Unit price, visualized and range, nm application (ug/mL) or Storage equipment Acute dose & cost method as (i.e., precast lowest Limits (gel Toxicity and per gel absorb/emit or post-gel) dilution documentation) Aquatic (abs/em) reported Toxicity Ethidium dsDNA 290 nm agarose 0.2ng-0.5ng May be UV Mutagenic with Managed Approx. $30 Bromide 1 ssDNA 605 nm stored at Transilluminator S9 activation as for 10mL of RNA acrylamide room of Salmonella hazardous 10mg/mL temperature; Polaroid 667 TA98 and waste solution; use PCR indefinite black & white TA1537 0.5µg/mL for storage strains agarose gel; yields 5,000 LD 50 , rat (oral): 40-mL gels, 1503 mg/kg $0.006/gel. (slightly toxic) Aquatic toxicity : LC 50 not available, MSDS indicates “may cause long- term adverse effects on aquatic environment” SYBR Safe 2 dsDNA 280/502 nm agarose Comparable Keep away UV Weakly Approved $53.75 for ssDNA 530 nm to ethidium from heat Transilluminator mutagenic with by MWRA 10,000X acrylamide bromide and light; S9 activation for drain SYBR Safe stable for blue (vis) light of Salmonella disposal, in DMSO, pre-cast approx. 6 transilluminator TA98 and May 2005 400µL; 4µL months (SafeImager™ TA1537 for 40 mL post-gel when stored recommended) strains gel = at room $0.5375 per temperature. laser scanner LD 50 , rat -

SYBR Safe Case Study
Replacing Ethidium Bromide in an Undergraduate Laboratory: SYBR Safe® Case Study March 2006 What is Ethidium Bromide and Why is it Used? Ethidium bromide (CAS #1239-45-8), or C21H20BrN3, is used in a number of laboratories, including those at MIT, for identifying DNA bands in samples that are loaded onto agarose gels. Ethidium bromide, commonly referred to as EtBr, binds to DNA. When placed under ultraviolet light, the EtBr-stained DNA bands fluoresce, allowing for the identification and visualization of nucleic acid bands. Ethidium bromide is considered an effective and relatively inexpensive technique for visualizing nucleic acid bands. Drawbacks of Ethidium Bromide Though effective and relatively inexpensive, ethidium bromide does have the following drawbacks for those handling the material in the lab: • it can be absorbed through the skin, irritating the eyes, mouth, and upper respiratory tract; • because of its tendency to intercalate in DNA bands, ethidium bromide is a powerful mutagen; • if handled indiscriminately in the lab, ethidium bromide can easily contaminate a large work area. When lab spaces are prepared for a move or for renovation, the space must be decontaminated of chemical, biological and radiological hazards. Because individual laboratories bear most, if not all, of the cost of decontaminating a lab, widespread ethidium bromide contamination may unnecessarily increase either the time or cost of lab preparation for moves or renovations; and • techniques for managing ethidium bromide waste are expensive - from a materials perspective, labor perspective, or both - or they beget more waste. Management of Ethidium Bromide Waste The United States Environmental Protection Agency (EPA) does not currently regulate ethidium bromide as a hazardous waste. -

PRICELIST-1920-FINAL.Pdf
INDEX Page No. MD Speech 01 Our Vision / Our Mission 02 Product Classification and Grade Information 03 Label Information 04 GHS Compliance 05 Technical Data Sheet and COA 06 Qualikems Product Range 07 ISO Certificate 08 - 09 Company Details 10 Ordering Information 11 Terms & Conditions 12 Rate List 13 - 52 Images of Lab / Plant / R & D 53 - 58 Rate List 59 -116 BELIEVING yourselfIN IS THE FIRST SECRET TO Success Dear Reader, The document you are holding is the result of work performed by the team of professionals of QUALIKEMS. It is the fruit of our teams extensive technical experience combine with the collaboration of our customers, who have offered us their valuable comments and proposals for improvement. At Qualikems, we have been working and investing for many years with our thoughts focused on the long term. Only thus can this comprehensive catalogue be kept up to date with the products you need. Our highly trained workforce, using state of the art technology, is the driving force behind the management of our modern factory, and our principal aim is to guarantee that the QUALIKEMS product range meets the conditions you require. QUALIKEMS reinforces industrial character and the path to progress we have continuously forged over the years. This path requires the responsible use of resources and the sustainability of our business activity. It is likewise requires and ability to keep on growing as the way to earn and to preserve our status as the leading supplier of laboratory reagents to our Clients Ashok Sahni Managing Director QUALIKEMS FINE CHEM PVT. -

162 Part 175—Indirect Food Addi
§ 174.6 21 CFR Ch. I (4–1–19 Edition) (c) The existence in this subchapter B Subpart B—Substances for Use Only as of a regulation prescribing safe condi- Components of Adhesives tions for the use of a substance as an Sec. article or component of articles that 175.105 Adhesives. contact food shall not be construed as 175.125 Pressure-sensitive adhesives. implying that such substance may be safely used as a direct additive in food. Subpart C—Substances for Use as (d) Substances that under conditions Components of Coatings of good manufacturing practice may be 175.210 Acrylate ester copolymer coating. safely used as components of articles 175.230 Hot-melt strippable food coatings. that contact food include the fol- 175.250 Paraffin (synthetic). lowing, subject to any prescribed limi- 175.260 Partial phosphoric acid esters of pol- yester resins. tations: 175.270 Poly(vinyl fluoride) resins. (1) Substances generally recognized 175.300 Resinous and polymeric coatings. as safe in or on food. 175.320 Resinous and polymeric coatings for (2) Substances generally recognized polyolefin films. as safe for their intended use in food 175.350 Vinyl acetate/crotonic acid copoly- mer. packaging. 175.360 Vinylidene chloride copolymer coat- (3) Substances used in accordance ings for nylon film. with a prior sanction or approval. 175.365 Vinylidene chloride copolymer coat- (4) Substances permitted for use by ings for polycarbonate film. 175.380 Xylene-formaldehyde resins con- regulations in this part and parts 175, densed with 4,4′-isopropylidenediphenol- 176, 177, 178 and § 179.45 of this chapter. -

Environmental Protection Agency § 117.3
Environmental Protection Agency § 117.3 (iii) Which are used or could be used (j) Process waste water means any for industrial purposes by industries in water which, during manufacturing or interstate commerce; processing, comes into direct contact (4) All impoundments of waters oth- with or results from the production or erwise defined as navigable waters use of any raw material, intermediate under this paragraph; product, finished product, byproduct, (5) Tributaries of waters identified in or waste product. paragraphs (i) (1) through (4) of this section, including adjacent wetlands; [44 FR 50776, Aug. 29, 1979, as amended at 58 and FR 45039, Aug. 25, 1993; 65 FR 30904, May 15, (6) Wetlands adjacent to waters iden- 2000] tified in paragraphs (i) (1) through (5) of this section (‘‘Wetlands’’ means § 117.2 Abbreviations. those areas that are inundated or satu- NPDES equals National Pollutant rated by surface or ground water at a Discharge Elimination System. RQ frequency and duration sufficient to equals reportable quantity. support, and that under normal cir- cumstances do support, a prevalence of § 117.3 Determination of reportable vegetation typically adapted for life in quantities. saturated soil conditions. Wetlands Each substance in Table 117.3 that is generally included playa lakes, listed in Table 302.4, 40 CFR part 302, is swamps, marshes, bogs, and similar assigned the reportable quantity listed areas such as sloughs, prairie potholes, in Table 302.4 for that substance. wet meadows, prairie river overflows, mudflats, and natural ponds): Provided, TABLE 117.3—REPORTABLE QUANTITIES That waste treatment systems (other OF HAZARDOUS SUBSTANCES DES- than cooling ponds meeting the cri- IGNATED PURSUANT TO SECTION 311 OF teria of this paragraph) are not waters THE CLEAN WATER ACT of the United States. -

A Study of the Decomposition of Some Aromatic Diazonium Hexafluorophosphate Salts
University of Windsor Scholarship at UWindsor Electronic Theses and Dissertations Theses, Dissertations, and Major Papers 1-1-1964 A study of the decomposition of some aromatic diazonium hexafluorophosphate salts. William A. Redmond University of Windsor Follow this and additional works at: https://scholar.uwindsor.ca/etd Recommended Citation Redmond, William A., "A study of the decomposition of some aromatic diazonium hexafluorophosphate salts." (1964). Electronic Theses and Dissertations. 6038. https://scholar.uwindsor.ca/etd/6038 This online database contains the full-text of PhD dissertations and Masters’ theses of University of Windsor students from 1954 forward. These documents are made available for personal study and research purposes only, in accordance with the Canadian Copyright Act and the Creative Commons license—CC BY-NC-ND (Attribution, Non-Commercial, No Derivative Works). Under this license, works must always be attributed to the copyright holder (original author), cannot be used for any commercial purposes, and may not be altered. Any other use would require the permission of the copyright holder. Students may inquire about withdrawing their dissertation and/or thesis from this database. For additional inquiries, please contact the repository administrator via email ([email protected]) or by telephone at 519-253-3000ext. 3208. A STUDY OF THE DECOMPOSITION OF SOME AROMATIC DIAZONIUM HEXAFLUOROPHOSPHATE SALTS BY WILLIAM A. REDMOND A Thesis Submitted to the Faculty of Graduate Studies through' the Department of Chemistry in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy at the University of Windsor Windsor, Ontario 1964 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. -

Environmental Protection Agency § 117.3
Environmental Protection Agency § 117.3 (4) Applicability date. This paragraph TABLE 117.3—REPORTABLE QUANTITIES OF (i) is applicable beginning on February HAZARDOUS SUBSTANCES DESIGNATED PUR- 6, 2020. SUANT TO SECTION 311 OF THE CLEAN (j) Process waste water means any WATER ACT—Continued water which, during manufacturing or Cat- RQ in pounds processing, comes into direct contact Material egory (kilograms) with or results from the production or use of any raw material, intermediate Ammonium benzoate ...................... D ...... 5,000 (2,270) Ammonium bicarbonate .................. D ...... 5,000 (2,270) product, finished product, byproduct, Ammonium bichromate ................... A ....... 10 (4.54) or waste product. Ammonium bifluoride ...................... B ....... 100 (45.4) Ammonium bisulfite ......................... D ...... 5,000 (2,270) [44 FR 50776, Aug. 29, 1979, as amended at 58 Ammonium carbamate .................... D ...... 5,000 (2,270) FR 45039, Aug. 25, 1993; 65 FR 30904, May 15, Ammonium carbonate ..................... D ...... 5,000 (2,270) 2000; 80 FR 37112, June 29, 2015; 83 FR 5208, Ammonium chloride ........................ D ...... 5,000 (2,270) Feb. 6, 2018] Ammonium chromate ...................... A ....... 10 (4.54) Ammonium citrate dibasic ............... D ...... 5,000 (2,270) Ammonium fluoborate ..................... D ...... 5,000 (2,270) § 117.2 Abbreviations. Ammonium fluoride ......................... B ....... 100 (45.4) NPDES equals National Pollutant Ammonium hydroxide ..................... C -

Ethidium Bromide Use and Disposal
Ethidium Bromide Use and Disposal NOTE: Ethidium bromide is a chemical and should NOT be treated or labeled as a biohazard Ethidium Bromide (EtBr), commonly used in research laboratories as a stain for the visualization of nucleic acids in electrophoresis gels, is a toxic chemical and a potent mutagen. When used in nucleic acid staining, ethidium bromide fluoresces a red-orange to pink color under ultraviolet light and with increased fluorescence when bound to double-stranded DNA. While it is not specifically regulated as a hazardous waste, the mutagenic properties may present health hazards and disposal concerns if it is not managed properly in the laboratory. Required PPE: Always wear a lab coat, gloves, and appropriate protective eyewear when handling ethidium bromide and/or ethidium bromide containing material. Proper skin and eye protection are also needed when an ultraviolet (UV) light source is used while working with ethidium bromide. Avoid exposing unprotected skin and eyes to intense UV sources. A face shield is suggested if the UV source is pointing upwards. Guidelines for Ethidium Bromide Disposal: Stock Solutions: Stock solutions of ethidium bromide typically contain higher concentrations of ethidium bromide (approximately 10 mg/ml). Contact the Office of Environmental Safety & Services for collection and proper disposal of all unwanted stock solutions of ethidium bromide. Electrophoresis Gels and Buffers: Solid ethidium bromide waste (e.g., gels) typically contains 3 –5 ug/ml of ethidium bromide. Liquid ethidium bromide -

Come-Back of Phenanthridine and Phenanthridinium Derivatives in the 21St Century
Come-back of phenanthridine and phenanthridinium derivatives in the 21st century Lidija-Marija Tumir, Marijana Radić Stojković and Ivo Piantanida* Review Open Access Address: Beilstein J. Org. Chem. 2014, 10, 2930–2954. Laboratory for Study of Interactions of Biomacromolecules, Division of doi:10.3762/bjoc.10.312 Organic Chemistry and Biochemistry, Ruđer Bošković Institute, Bijenička cesta 54, PO Box 180, HR-10002 Zagreb, Croatia Received: 17 July 2014 Accepted: 21 November 2014 Email: Published: 10 December 2014 Ivo Piantanida* - [email protected] This article is part of the Thematic Series "Nucleic acid chemistry". * Corresponding author Guest Editor: H.-A. Wagenknecht Keywords: ds-DNA and ds-RNA binding; intercalation; minor groove binding; © 2014 Tumir et al; licensee Beilstein-Institut. nucleic acids; organic synthesis; phenanthridine; phenanthridinium License and terms: see end of document. Abstract Phenanthridine derivatives are one of the most intensively studied families of biologically active compounds with efficient DNA binding capability. Attracting attention since DNA structure discovery (1960s), they were early recognized as a symbol of DNA intercalative binding, for many decades applied as gold-standard DNA- and RNA-fluorescent markers (ethidium bromide), probes for cell viability (propidium iodide), but also “ill-famed” for various toxic (genotoxic) and mutagenic effects. After two decades of low interest, the discovery of phenanthridine alkaloids and new studies of antiparasitic/antitumor properties of phenanthridine derivatives resulted in the strong increase of the scientific interest about the turn of this century. Here are summarized phenanthri- dine-related advances in the 21st century (2000-present period) with emphasis on the supramolecular interactions and bioorganic chemistry, as well as novel or improved synthetic approaches.