Optimization the Continuous Distillation Process of an Aqueous Ethanol Mixture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Innovation in Continuous Rectification for Tequila Production
processes Communication Innovation in Continuous Rectification for Tequila Production Estarrón-Espinosa Mirna, Ruperto-Pérez Mariela, Padilla-de la Rosa José Daniel * and Prado-Ramírez Rogelio * Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ), Av. Normalistas No. 800, C.P. 44720 Guadalajara, Jalisco, Mexico; [email protected] (E.-E.M.); [email protected] (R.-P.M.) * Correspondence: [email protected] (P.-d.l.R.J.D.); [email protected] (P.-R.R.); Tel.: +33-33455200 (P.-d.l.R.J.D.) Received: 23 March 2019; Accepted: 6 May 2019; Published: 14 May 2019 Abstract: In this study, a new process of continuous horizontal distillation at a pilot level is presented. It was applied for the first time to the rectification of an ordinario fraction obtained industrially. Continuous horizontal distillation is a new process whose design combines the benefits of both distillation columns, in terms of productivity and energy savings (50%), and distillation stills in batch, in terms of the aromatic complexity of the distillate obtained. The horizontal process of continuous distillation was carried out at the pilot level in a manual mode, obtaining five accumulated fractions of distillate that were characterized by gas chromatography (GC-FID). The tequila obtained from the rectification process in this new continuous horizontal distillation process complies with the content of methanol and higher alcohols regulated by the Official Mexican Standard (NOM-006-SCFI-2012). Continuous horizontal distillation of tequila has potential energy savings of 50% compared to the traditional process, besides allowing products with major volatile profiles within the maximum limits established by the regulation for this beverage to be obtained. -

Page2 Sidebar 1.Pdf
Headquarters & Forge Americas Office [email protected] [email protected] +49 (0) 7161 / 97830 215.242.6806 +49 (0) 7161 / 978321 fax fax 215.701.9725 artisan distilling systems 600 liter whiskey still the fine art of distillery technology Germany’s oldest distillery fabricator, since 1869, combining traditional family craftsmanship with leading eau-de-vie distillery innovations and technologies. Meticulously custom-crafted artisan copper pot still systems for all the great distilling traditions, and efficient continuous plants, grappa distillery in copper and stainless steel, for all capacities and applications: 450 liter artisan pot stills – brandy & vodka vodka, whiskey, eaux-de-vie, brandy, rum, gin, grappa, tequila, aguardientes… 1000 liter artisan vodka system 650 liter system with CADi automation continuous mash stripping column C. CARL Ziegelstraße 21 Americas Office Brewing & Distilling Ing. GmbH D-73033 Göppingen PO Box 4388 Technologies Corp. www.christiancarl.com Germany Philadelphia, PA 19118-8388 www.brewing-distilling.com CARL artisan distillery systems the fine art of distillery technology CARL custom-builds each distillery to order in our family shop near Stuttgart in Swabia, with the attention and care of crafting a finely- tuned instrument. All-the-while, we stay focused on the continued development of our distillery technology. There are always new ideas and realizations, such our the in-house developed CARL CADi distillery automation or our patented aroma bubble plate technologies, Our innovations have fostered CARL’s nearly 140 years of family tradition and experience as Germany’s oldest and most respected distillery fabricator, with thousands of successful commissions worldwide. form and function Our diverse customers, from small farmers to winemakers to brewers to large spirits houses, show great enthusiasm and appreciation for the aesthetics and functionality of a CARL distillery: its design, its form, classic and intuitively easy to understand, clear in conception. -

Distillation 6
CHAPTER Energy Considerations in Distillation 6 Megan Jobson School of Chemical Engineering and Analytical Science, The University of Manchester, Manchester, UK CHAPTER OUTLINE 6.1 Introduction to energy efficiency ....................................................................... 226 6.1.1 Energy efficiency: technical issues................................................... 227 6.1.1.1 Heating.................................................................................... 227 6.1.1.2 Coolingdabove ambient temperatures...................................... 229 6.1.1.3 Coolingdbelow ambient temperatures ...................................... 230 6.1.1.4 Mechanical or electrical power.................................................. 232 6.1.1.5 Summarydtechnical aspects of energy efficiency ..................... 233 6.1.2 Energy efficiency: process economics............................................... 233 6.1.2.1 Heating.................................................................................... 233 6.1.2.2 Cooling..................................................................................... 235 6.1.2.3 Summarydprocess economics and energy efficiency ............... 237 6.1.3 Energy efficiency: sustainable industrial development........................ 237 6.2 Energy-efficient distillation ............................................................................... 237 6.2.1 Energy-efficient distillation: conceptual design of simple columns...... 238 6.2.1.1 Degrees of freedom in design .................................................. -

Modelling of Crude Oil Distillation
Modelling of Crude Oil Distillation Modellering av råoljedestillation JENN Y BEATRIC E SOUC K K T H C h em i ca l S ci en c e and E n gi n e e r i n g Master’s Thesis in Chemical Engineering Stockholm, Sweden 2012 " One never notices what has been done; one can only see what remains to be done" (Marie Curie, 1867-1934) 1 Sammanfattning Under de föhållanden som reservoarens miljö erbjuder, definieras en petroleumvätska av dess termodynamiska och volymetriska egenskaper och av dess fysikalisk-kemiska egenskaper. För att korrekt simulera bearbetningen av dessa vätskor under produktion, deras beteende modelleras från experimentella data Med tillkomsten av nya regler och oflexibilitet som finns på tullbestämmelser vid gränserna idag, har forskningscenter stora svårigheter att få större mängder prover levererade. Av den anledningen, trots att det finns flera metoder för att karakterisera de olika komponenterna av råolja, tvingas laboratorier att vända sig mer och mer till alternativa analysmetoder som kräver mindre provvolymer: mikrodestillation, gaskromatografi, etc. Mikrodestillation, som är en snabb och helt datoriserad teknik, visar sig kunna ersätta standarddestillation för analys av flytande petroleumprodukter. Fördelar med metoden jämfört med standarddestillering är minskad arbetstidsåtgång med minst en faktor 4. Därtill krävs endast en [24] begränsad provvolym (några mikroliter) i jämförelse med standarddestillation. Denna rapport syftar till att skapa en enkel modell som kan förutsäga avkastningskurvan av fysisk destillation, utan att använda mikrodestillationsteknik. De resultat som erhölls genom gaskromatografiska analyser möjliggjorde modelleringen av det vätskebeteendet hos det analyserade provet. Efter att ha identifierat och behandlat praktiskt taget alla viktiga aspekter av mikro destillation genom simuleringar med PRO/II, fann jag att, oberoende av inställningen och den termodynamiska metod som används, det alltid finns stora skillnader mellan simulering och mikro destillation. -

Alembic Pot Still
ALEMBIC POT STILL INSTRUCTION MANUAL CAN BE USED WITH THE GRAINFATHER OR T500 BOILER SAFETY Warning: This system produces a highly flammable liquid. PRECAUTION: • Always use the Alembic Pot Still System in a room with adequate ventilation. • Never leave the Alembic Pot Still system unattended when operating. • Keep the Alembic Pot Still system away from all sources of ignition, including smoking, sparks, heat, and open flames. • Ensure all other equipment near to the Alembic Pot Still system or the alcohol is earthed. • A fire extinguishing media suitable for alcohol should be kept nearby. This can be water fog, fine water spray, foam, dry powder, carbon dioxide, sand or dolomite. • Do not boil dry. In the event the still is boiled dry, reset the cutout button under the base of the still. In the very unlikely event this cutout fails, a fusible link gives an added protection. IN CASE OF SPILLAGE: • Shut off all possible sources of ignition. • Clean up spills immediately using cloth, paper towels or other absorbent materials such as soil, sand or other inert material. • Collect, seal and dispose accordingly • Mop area with excess water. CONTENTS Important points before getting started ............................................................................... 3 Preparing the Alembic Pot Still ................................................................................................. 5 Distilling a Whiskey, Rum or Brandy .......................................................................................7 Distilling neutral -

Minimum Reflux in Liquid–Liquid Extraction
17th European Symposium on Computer Aided Process Engineering – ESCAPE17 V. Plesu and P.S. Agachi (Editors) © 2007 Elsevier B.V. All rights reserved. 1 Minimum Reflux in Liquid–Liquid Extraction Santanu Bandyopadhyaya and Calin-Cristian Cormosb aEnergy Systems Engineering, Indian Institute of Technology, Bombay, Powai, Mumbai 400 076, India, E-mail: [email protected] bDepartment of Chemical Engineering, Faculty of Chemistry and Chemical Engineering, University "Babeş-Bolyai", Arany Janos 11, Cluj-Napoca 400028, Romania, E-mail: [email protected] Abstract In a simple countercurrent arrangement of different stages of liquid–liquid extraction operation, the richest extract leaving the operation is in equilibrium with the feed. However, by using reflux it is possible to enrich the extract further. A simple counter current liquid–liquid extraction operation with reflux is analogous in its essentials to distillation. In this paper, the method based on Invariant Rectifying-Stripping (IRS) curves, originally proposed to calculate minimum reflux and minimum energy requirement in distillation, has been extended to liquid–liquid extraction. The equivalent IRS curves for a ternary liquid–liquid extraction predicts the feed location in the counter current process. It also predicts the minimum reflux requirement for a given separation and minimum amount of solvent required. Keywords: liquid extraction, IRS curves, feed location, minimum solvent. 1. Introduction Liquid–liquid extraction is a separation process that takes advantage of the distribution of a substance between two insoluble liquids phases [1]. Feed to be separated is mixed with extracting solvent to produce a solvent-rich phase, called extract, and a solvent-lean phase, called raffinate. -

Reflux Condensation in Narrow Rectangular Channels with Perforated Fins Nadia Souidi, André Bontemps
Reflux condensation in narrow rectangular channels with perforated fins Nadia Souidi, André Bontemps To cite this version: Nadia Souidi, André Bontemps. Reflux condensation in narrow rectangular channels with perforated fins. Applied Thermal Engineering, Elsevier, 2003, 23, pp.871-891. 10.1016/S1359-4311(03)00021-8. hal-00184135 HAL Id: hal-00184135 https://hal.archives-ouvertes.fr/hal-00184135 Submitted on 19 Feb 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Reflux condensation in narrow rectangular channels with perforated fins N. Souidi a, A. Bontemps b,* a GRETh-CEA Grenoble, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France b LEGI-GRETh, Universitee Joseph Fourier, 17 Rue des Martyrs, 38054 Grenoble Cedex 9, France Reflux condensation is an industrial process that aims to reduce the content of the less volatile com- ponent or to eliminate the non-condensable phase of a vapour mixture, by the means of separation. Separation consists in condensing the less volatile phase and to recover the condensate while simulta- neously, the non-condensable species are recuperated at the top of the system. Compact plate-fin heat exchangers can be used in gas separation processes. -

Computer Simulation of a Multicomponent, Multistage Batch Distillation Process
COMPUTER SIMULATION OF A MULTICOMPONENT, MULTISTAGE BATCH DISTILLATION PROCESS By BRUCE EARL BAUGHER /J Bachelor of Science in Chemical Engineering Oklahoma State University Stillwater, Oklahoma 1985 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE July, 1988 \\,~s~ ~ \'\~~ B~~~to' <:.o?. ·;)._, ~ r COMPUTER SIMULATION OF A MULTICOMPONENT, MULTISTAGE BATCH DISTILLATION PROCESS Thesis Approved: Thesis Advisor U.bJ ~~- ~MJYl~ Dean of the GradU~te College ABSTRACT The material and energy balance equations for a batch distillation column were derived and a computer simulation was developed to solve these equations. The solution follows a modified Newton-Raphson approach for inverting and solving the matrices of material and energy balance equations. The model is unique in that it has been designed to handle hypothetical hydrocarbon components. The simulation can handle columns up to 50 trays and systems of up to 100 components. The model has been used to simulate True Boiling Point (TBP) diagrams for a variety of crude oils. This simulation is also applicable to simple laboratory batch distillations. The model was designed to accurately combine data files to simulate actual crude blending procedures. The model will combine files and calculate the results quickly and easily. The simulation involves removing material from the column at steady-state. The removal fraction is made small enough to approach continuity. This simulation can be adapted for use on microcomputers, although it will require extensive computation time. ACKNOWLEDGMENTS It has been a great pleasure to work with my thesis advisor, Dr. -

Distillation Handbook
I distillation terminology To provide a better understanding of the distillation process, the following briefly explains the terminology most often encountered. SOLVENT RECOVERY The term "solvent recovery" often has been a somewhat vague label applied to the many and very different ways in which solvents can be reclaimed by industry. One approach employed in the printing and coatings industries is merely to take impure solvents containing both soluble and insoluble particles and evapo- rate the solvent from the solids. For a duty of this type, APV offers the Paraflash evaporator, a compact unit which combines a Paraflow plate heat exchanger and a small separator. As the solvent ladened liquid is recirculated through the heat exchanger, it is evaporated and the vapor and liquid separated. This will recover a solvent but it will not separate solvents if two or more are present. Afurther technique is available to handle an air stream that carries solvents. By chilling the air by means of vent condensers or refrigeration equipment, the solvents can be removed from the condenser. Solvents also can be recovered by using extraction, adsorption, absorption, and distillation methods. S 0 LV E NT EXT R ACT I0 N Essentially a liquid/liquid process where one liquid is used to extract another from a secondary stream, solvent extraction generally is performed in a column somewhat similar to a normal distillation column. The primary difference is that the process involves two liquids instead of liquid and vapor. During the process, the lighter (i.e., less dense) liquid is charged to the base of the column and rises through packing or trays while the more dense liquid descends. -

Building a Home Distillation Apparatus
BUILDING A HOME DISTILLATION APPARATUS A Step by Step Guide Building a Home Distillation Apparatus i BUILDING A HOME DISTILLATION APPARATUS Foreword The pages that follow contain a step-by-step guide to building a relatively sophisticated distillation apparatus from commonly available materials, using simple tools, and at a cost of under $100 USD. The information contained on this site is directed at anyone who may want to know more about the subject: students, hobbyists, tinkers, pure water enthusiasts, survivors, the curious, and perhaps even amateur wine and beer makers. Designing and building this apparatus is the only subject of this manual. You will find that it confines itself solely to those areas. It does not enter into the domains of fermentation, recipes for making mash, beer, wine or any other spirits. These areas are covered in detail in other readily available books and numerous web sites. The site contains two separate design plans for the stills. And while both can be used for a number of distillation tasks, it should be recognized that their designs have been optimized for the task of separating ethyl alcohol from a water-based mixture. Having said that, remember that the real purpose of this site is to educate and inform those of you who are interested in this subject. It is not to be construed in any fashion as an encouragement to break the law. If you believe the law is incorrect, please take the time to contact your representatives in government, cast your vote at the polls, write newsletters to the media, and in general, try to make the changes in a legal and democratic manner. -

Batch Distillation of Spirits: Experimental Study and Simulation
Research article Received: 5 April 2018 Revised: 11 January 2019 Accepted: 15 January 2019 Published online in Wiley Online Library (wileyonlinelibrary.com) DOI 10.1002/jib.560 Batch distillation of spirits: experimental study and simulation of the behaviour of volatile aroma compounds Adrien Douady,1 Cristian Puentes,1 Pierre Awad1,2 and Martine Esteban-Decloux1* This paper focuses on the behaviour of volatile compounds during batch distillation of wine or low wine, in traditional Charentais copper stills, heated with a direct open flame at laboratory (600 L) and industrial (2500 L) scale. Sixty-nine volatile compounds plus ethanol were analysed during the low wine distillation in the 600 L alembic still. Forty-four were quantified and classified according to their concentration profile in the distillate over time and compared with previous studies. Based on the online re- cording of volume flow, density and temperature of the distillate with a Coriolis flowmeter, distillation was simulated with ProSim® BatchColumn software. Twenty-six volatile compounds were taken into account, using the coefficients of the ‘Non- Random Two Liquids’ model. The concentration profiles of 18 compounds were accurately represented, with slight differences in the maximum concentration for seven species together with a single compound that was poorly represented. The distribution of the volatile compounds in the four distillate fractions (heads, heart, seconds and tails) was well estimated by simulation. Fi- nally, data from wine and low wine distillations in the large-scale alembic still (2500 L) were correctly simulated, suggesting that it was possible to adjust the simulation parameters with the Coriolis flowmeter recording and represent the concentration pro- files of most of the quantifiable volatile compounds. -
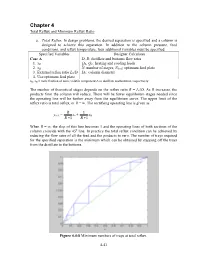
Minimum Reflux Ratio
Chapter 4 Total Reflux and Minimum Reflux Ratio a. Total Reflux . In design problems, the desired separation is specified and a column is designed to achieve this separation. In addition to the column pressure, feed conditions, and reflux temperature, four additional variables must be specified. Specified Variables Designer Calculates Case A D, B: distillate and bottoms flow rates 1. xD QR, QC: heating and cooling loads 2. xB N: number of stages, Nfeed : optimum feed plate 3. External reflux ratio L0/D DC: column diameter 4. Use optimum feed plate xD, xB = mole fraction of more volatile component A in distillate and bottoms, respectively The number of theoretical stages depends on the reflux ratio R = L0/D. As R increases, the products from the column will reduce. There will be fewer equilibrium stages needed since the operating line will be further away from the equilibrium curve. The upper limit of the reflux ratio is total reflux, or R = ∞. The rectifying operating line is given as R 1 yn+1 = xn + xD R +1 R +1 When R = ∞, the slop of this line becomes 1 and the operating lines of both sections of the column coincide with the 45 o line. In practice the total reflux condition can be achieved by reducing the flow rates of all the feed and the products to zero. The number of trays required for the specified separation is the minimum which can be obtained by stepping off the trays from the distillate to the bottoms. Figure 4.4-8 Minimum numbers of trays at total reflux.