X-Ray Fluorescence Applications in Mudrock Characterization: Investigations Into Middle Devonian Stratigraphy, Appalachian Basin, USA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lacustrine Massive Mudrock in the Eocene Jiyang Depression, Bohai Bay Basin, China: Nature, Origin and Significance
Marine and Petroleum Geology 77 (2016) 1042e1055 Contents lists available at ScienceDirect Marine and Petroleum Geology journal homepage: www.elsevier.com/locate/marpetgeo Research paper Lacustrine massive mudrock in the Eocene Jiyang Depression, Bohai Bay Basin, China: Nature, origin and significance * Jianguo Zhang a, b, Zaixing Jiang a, b, , Chao Liang c, Jing Wu d, Benzhong Xian e, f, Qing Li e, f a College of Energy, China University of Geosciences, Beijing 100083, China b Institute of Earth Science, China University of Geosciences, Beijing 100083, China c School of Geosciences, China University of Petroleum (east China), Qingdao 266580, China d Petroleum Exploration and Production Research Institute, SINOPEC, Beijing 100083, China e College of Geosciences, China University of Petroleum, Beijing 102249, China f State Key Laboratory of Petroleum Resources and Prospecting, Beijing 102249, China article info abstract Article history: Massive mudrock refers to mudrock with internally homogeneous characteristics and an absence of Received 13 May 2016 laminae. Previous studies were primarily conducted in the marine environment, while notably few Accepted 6 August 2016 studies have investigated lacustrine massive mudrock. Based on core observation in the lacustrine Available online 8 August 2016 environment of the Jiyang Depression, Bohai Bay Basin, China, massive mudrock is a common deep water fine-grained sedimentary rock. There are two types of massive mudrock. Both types are sharply delin- Keywords: eated at the bottom and top contacts, abundant in angular terrigenous debris, and associated with Massive mudrock oxygen-rich (higher than 2 ml O /L H O) but lower water salinities in comparison to adjacent black Muddy mass transportation deposit 2 2 Turbiditic mudrock shales. -

Colorado Plateau Coring Project, Phase I (CPCP-I)
Science Reports Sci. Dril., 24, 15–40, 2018 https://doi.org/10.5194/sd-24-15-2018 © Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Colorado Plateau Coring Project, Phase I (CPCP-I): a continuously cored, globally exportable chronology of Triassic continental environmental change from western North America Paul E. Olsen1, John W. Geissman2, Dennis V. Kent3,1, George E. Gehrels4, Roland Mundil5, Randall B. Irmis6, Christopher Lepre1,3, Cornelia Rasmussen6, Dominique Giesler4, William G. Parker7, Natalia Zakharova8,1, Wolfram M. Kürschner9, Charlotte Miller10, Viktoria Baranyi9, Morgan F. Schaller11, Jessica H. Whiteside12, Douglas Schnurrenberger13, Anders Noren13, Kristina Brady Shannon13, Ryan O’Grady13, Matthew W. Colbert14, Jessie Maisano14, David Edey14, Sean T. Kinney1, Roberto Molina-Garza15, Gerhard H. Bachman16, Jingeng Sha17, and the CPCD team* 1Lamont-Doherty Earth Observatory of Columbia University, Palisades, NY 10964, USA 2Department of Geosciences, University of Texas at Dallas, Richardson, TX 75080, USA 3Earth and Planetary Sciences, Rutgers University, Piscataway, NJ 08854, USA 4Department of Geosciences, University of Arizona, Tucson, AZ 85721, USA 5Berkeley Geochronology Center, 2455 Ridge Rd., Berkeley CA 94709, USA 6Natural History Museum of Utah and Department of Geology & Geophysics, University of Utah, Salt Lake City, UT 84108, USA 7Petrified Forest National Park, Petrified Forest, AZ 86028, USA 8Department of Earth and Atmospheric Sciences, Central Michigan University, Mount Pleasant, MI 48859, USA 9Department of Geosciences, University of Oslo, P.O. Box 1047, Blindern, Oslo 0316, Norway 10MARUM Center for Marine Environmental Sciences, University of Bremen, Bremen, Germany 11Earth and Environmental Sciences, Rensselaer Polytechnic Institute (RPI), Troy, NY 12180, USA 12National Oceanography Centre, Southampton, University of Southampton, Southampton, SO17 1BJ, UK 13Continental Scientific Drilling Coordination Office and LacCore Facility, N.H. -

Slate Shale/Mudrock Regional 060(240)±20/70±10SE 020(200)±20/20±10NW No 060(240)±20/70±10SE 120(300)±20/20±10SW Yes No Q
Name: Reg. lab day: M Tu W Th F Geology 1013 Field trip to Black River Valley (Lab #7, Answer Key) Drive south on Gaspereau Avenue and stop at the Highway 101 overpass. Stop 1: HALIFAX GROUP A: Go to the north side of the overpass. slate a) Name the rock exposed in this outcrop. b) What was the original rock before metamorphism? Shale/mudrock c) Was the metamorphism regional or contact? regional d) Use the compass to measure strike & dip of cleavage. 060(240)±20/70±10SE e) Use the compass to measure strike & dip of bedding. 020(200)±20/20±10NW f) Is cleavage parallel to bedding? no g) Plot cleavage and bedding on the attached map using the proper symbols. B: Go to the south side of the overpass. 060(240)±20/70±10SE a) Use the compass to measure strike & dip of cleavage. 120(300)±20/20±10SW b) Use the compass to measure strike & dip of bedding. c) Is the cleavage orientation similar to that in (d) above? yes d) Is the bedding orientation similar to that in (e) above? no Drive on through Gaspereau to White Rock. Go through the intersection in White Rock and stop in the big quarry on the right. Stop 2: WHITE ROCK FORMATION quartzite a) Name the rock. quartz sandstone b) What was the original rock before metamorphism? c) This rock unit is more resistant to weathering than the Halifax Formation. Suggest reasons why. 1. composition (hard, chemically inert mineral) 2. no foliation (no planes of weakness) Black River Lab – Answer Key Page 2 of 4 Drive back through White Rock, turn south, and stop at the parking area at the Gaspereau River bridge. -

Clippety Clop), Kwelera, East London, Great Kei Municipality, Eastern Cape
PALAEONTOLOGICAL ASSESSMENT: COMBINED FIELD ASSESSMENT AND DESKTOP STUDY Proposed development of Portion 3 of Farm 695 (Clippety Clop), Kwelera, East London, Great Kei Municipality, Eastern Cape. JOHN E. ALMOND (PhD, Cantab) Natura Viva cc, PO Box 12410 Mill Street, CAPE TOWN 8010, RSA. [email protected] October 2011 1. SUMMARY The proposed holiday housing development on Portion 3 of Farm 695 (Clippety Clop), Kwelera, East London, is situated on the northern banks of the tidal Kwelera River, some 20 km northeast of East London, Eastern Cape. The development footprint is largely underlain by Late Permian continental sediments of the Adelaide Subgroup (Lower Beaufort Group, c. 253-251 million years old). These rocks are overlain by Early Triassic sandstones of the Katberg Formation (Tarkastad Subgroup) that build the cliffs and higher ground to the northeast. South of the river the Beaufort Group sediments are intruded and baked by Early Jurassic igneous intrusions of the Karoo Dolerite Suite. The Balfour Formation fluvial sediments are potentially fossiliferous, having yielded elsewhere a wide range of terrestrial vertebrates (bones and teeth of pareiasaurs, therapsids, amphibians et al.), bivalves, trace fossils and vascular plants. The overall impact of this project on local palaeontological heritage is likely to be very minor, however, because the potentially fossiliferous Beaufort Group sediments here are (a) deeply weathered, (b) sparsely fossiliferous, (c) have probably been extensively baked by nearby dolerite intrusions, and (d) are mostly covered with a thick (> 3m) mantle of fossil-poor alluvium. No fossils were observed within good exposures of the Balfour Formation rocks at the coast and in excellent roadcuts inland. -
Metamorphic Rocks Specimen Guide
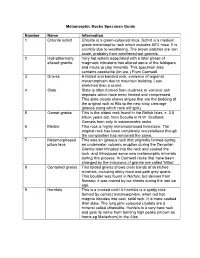
Metamorphic Rocks Specimen Guide Number Name Information 1 Chlorite schist Chlorite is a green-coloured mica. Schist is a medium grade metamorphic rock which includes 50% mica. It is crumbly due to weathering. The brown patches are iron oxide, probably from weathered-out garnets. 2 Hydrothermally Very hot waters associated with a later phase of altered granite magmatic intrusions has altered some of the feldspars and micas to clay minerals. This specimen also contains cassiterite (tin ore.) From Cornwall. 3 Gneiss A folded and banded rock, evidence of regional metamorphism due to mountain building. Less stretched than a schist. 4 Slate Slate is often formed from mudrock or volcanic ash deposits which have been heated and compressed. This slate clearly shows stripes that are the bedding of the original rock at 90o to the new slaty cleavage (planes along which rock will split) 5 Garnet gneiss This is the oldest rock found in the British Isles, c. 3.8 billion years old, from Scourie in N.W. Scotland. Garnets form only in metamorphic rocks. 6 Marble This rock is highly metamorphosed limestone. The original rock has been completely recrystallised though the composition has remained the same. 7 Metamorphosed This was an igneous rock that originally formed during pillow lava an underwater volcanic eruption during the Devonian. Granite later intruded into the rock and cooked the rock, and introduced some new metamorphic minerals during this process. In Cornwall rocks that have been changed by the intrusions of granite are called “killas”. 8 Contorted gneiss This folded gneiss shows clear bands of stretched minerals, including shiny mica and pale grey quartz. -

International Journal of Coal Geology 165 (2016) 76–89
International Journal of Coal Geology 165 (2016) 76–89 Contents lists available at ScienceDirect International Journal of Coal Geology journal homepage: www.elsevier.com/locate/ijcoalgeo Microstructures of Early Jurassic (Toarcian) shales of Northern Europe M.E. Houben a,⁎,A.Barnhoornb, L. Wasch c, J. Trabucho-Alexandre a,C.J.Peacha,M.R.Drurya a Faculty of Geosciences, Utrecht University, PO-box 80.021, 3508TA Utrecht, The Netherlands b Faculty of Civil Engineering & Geosciences, Delft University of Technology, PO-box 5048, 2600GA Delft, The Netherlands c TNO, Princetonlaan 6, 3584 CB Utrecht, The Netherlands article info abstract Article history: The Toarcian (Early Jurassic) Posidonia Shale Formation is a possible unconventional gas source in Northern Received 7 January 2016 Europe and occurs within the Cleveland Basin (United Kingdom), the Anglo-Paris Basin (France), the Lower Sax- Received in revised form 4 August 2016 ony Basin and the Southwest Germany Basin (Germany), and the Roer Valley Graben, the West Netherlands Accepted 4 August 2016 Basin, Broad Fourteens Basin, the Central Netherlands Basin and the Dutch Central Graben in The Netherlands. Available online 06 August 2016 Outcrops can be found in the United Kingdom and Germany. Since the Posidonia Shale Formation does not out- crop in the Netherlands, sample material suitable for experimental studies is not easily available. Here we have Keywords: Posidonia Shale investigated lateral equivalent shale samples from six different locations across Northern Europe (Germany, Whitby Mudstone The Netherlands, The North sea and United Kingdom) to compare the microstructure and composition of Clay microstructure Toarcian shales. The objective is to determine how homogeneous or heterogeneous the shale deposits are across Ion-beam polishing the basins, using a combination of Ion Beam polishing, Scanning Electron Microscopy and X-ray diffraction. -

Rehabilitation of National Route R61 (Section 3, Km 24.2 to Km 75) Between Cradock and Tarkastad, Eastern Cape
PALAEONTOLOGICAL HERITAGE STUDY: COMBINED DESKTOP AND FIELD-BASED ASSESSMENT Rehabilitation of National Route R61 (Section 3, km 24.2 to km 75) between Cradock and Tarkastad, Eastern Cape John E. Almond PhD (Cantab.) Natura Viva cc, PO Box 12410 Mill Street, Cape Town 8010, RSA [email protected] February 2013 1. SUMMARY The South African National Roads Agency Limited (SANRAL) is proposing to rehabilitate Section 3 of the National Route R61 (km 24.2 to km 75) between Cradock and Tarkastad, Eastern Cape. The project involves widening of the roadway and of all stormwater structures along the route. Road material is to be sourced from five new or existing borrow pits and one hard rock quarry. A Phase 1 palaeontological heritage assessment for the road project has been commissioned by Arcus GIBB (Pty) Ltd in accordance with the requirements of the National Heritage Resources Act (Act 25 of 1999). Section 3 of the R61 traverses the outcrop area of continental sedimentary rocks of the Upper Beaufort Group (Tarkastad Subgroup, Karoo Supergroup) of Early to Middle Triassic age. These are cut and baked by numerous dolerite intrusions of the Karoo Dolerite Suite of Early Jurassic age. Towards Cradock (Graaff-Reinet and Middelburg 1: 250 000 sheet areas) the sedimentary bedrocks belong to the sandstone-dominated Katberg Formation that was deposited in arid braided fluvial settings following the catastrophic end-Permian mass extinction event. Further east towards Tarkastad (Queenstown and King William’s Town 1: 250 000 sheet areas) the sedimentary bedrocks are assigned to the slightly younger Burgersdorp Formation comprising recessive-weathering reddish mudrocks and braided river channel sandstones. -

Aluminosilicate Diagenesis in a Tertiary Sandstone-Mudrock Sequence from the Central North Sea, Uk
Clay Minerals (1996) 31, 523–536 ALUMINOSILICATE DIAGENESIS IN A TERTIARY SANDSTONE-MUDROCK SEQUENCE FROM THE CENTRAL NORTH SEA, UK J. M. HUGGETT Department of Geology, Imperial College of Science, Technology and Medicine, London, SW7 2BP, UK (Received 24 November 1995; revised 20 March 1996) ABSTRACT: Mudrocks and sandstones from the Palaeocene of the central North Sea have been studied to assess the petrology, diagenesis and extent of any chemical interaction between the two lithologies. Authigenicand detrital minerals have been distinguished using a variety of electron microscope techniques. Small but significant quantities of authigenic minerals, which would not be detected by conventional petrographic tools, have been detected through the use of high-resolution electron beam techniques. Sandstone mineralogy has been quantified by point counting, and mudrock mineralogy semi-quantified by XRD. The detrital and authigenicmineralogy in the sandstone is almost identical to that found in the mudrock. The principal difference is in the relative proportions. Qualitative mass balance suggests that cross-formational flow has not been significant in either clay or quartz diagenesis. Researchers attempting to quantify the diagenetic SAMPLE MATERIAL relationship between mudrocks and sandstones have concluded that mudrocks act as closed The sampled interval is a cored Palaeocene systems (e.g. Hower et al., 1976; Pearson & sandstone dominated sequence from a well in the Small, 1988; Totten & Blatt, 1993) or that UK sector of the central North Sea. The overall reactants move freely between the two lithologies ratio of sandstone to mudrock is high, *84%, and (e.g. Curtis, 1978; Boles & Franks, 1979; individual mudrock beds average *0.3 m thick. -

The Impact of Coastal Geodynamic Processes on the Distribution of Trace Metal Content in Sandy Beach Sediments, South-Eastern Baltic Sea Coast (Lithuania)
applied sciences Article The Impact of Coastal Geodynamic Processes on the Distribution of Trace Metal Content in Sandy Beach Sediments, South-Eastern Baltic Sea Coast (Lithuania) Dovile˙ Karloniene˙ 1,*, Donatas Pupienis 1,2 , Darius Jarmalaviˇcius 2, Aira Dubikaltiniene˙ 1 and Gintautas Žilinskas 2 1 Faculty of Chemistry and Geosciences, Naugarduko st. 24, LT 03225 Vilnius, Lithuania; [email protected] (D.P.); [email protected] (A.D.) 2 Nature Research Centre, Akademijos st. 2, LT 08412 Vilnius, Lithuania; [email protected] (D.J.); [email protected] (G.Ž.) * Correspondence: [email protected] Featured Application: Geochemical analysis can provide valuable information about the local and regional patterns of sediment transport, distribution, provenance, and coasts’ conditions. Abstract: Sandy coasts are one of the most dynamic spheres; continuously changing due to natural processes (severe weather and rising water levels) and human activities (coastal protection or port construction). Coastal geodynamic processes lead to beach sediment erosion or accumulation. The coast’s dynamic tendencies determine the changes in the volume of beach sediments; grain size; mineralogical; and geochemical composition of sediments. In addition to lithological and mineralogical analysis of sediments, geochemical analysis can provide valuable information about Citation: Karloniene,˙ D.; Pupienis, the local and regional patterns of sediment transport, distribution, provenance, and coasts’ conditions. D.; Jarmalaviˇcius,D.; Dubikaltiniene,˙ The study aims to assess trace metals’ temporal and spatial distribution determined in the sandy beach A.; Žilinskas, G. The Impact of sediments along the south-eastern Baltic Sea coast (Lithuania) during 2011–2018. The Lithuanian Coastal Geodynamic Processes on the seacoast is divided into two parts: mainland and spit coast. -

Stratigraphic Completeness and Resolution in an Ancient Mudrock Succession
Sedimentology (2018) 65, 1875–1890 doi: 10.1111/sed.12450 Stratigraphic completeness and resolution in an ancient mudrock succession DAVID B. KEMP*, WESLEY T. FRASER† and KENTARO IZUMI‡ *School of Geosciences, University of Aberdeen, Old Aberdeen, Aberdeen AB24 3UE, UK (E-mail: [email protected]) †Geography, Department of Social Sciences, Oxford Brookes University, Oxford OX3 0BP, UK ‡Faculty and Graduate School of Education, Chiba University, 1-33 Yoyoi-cho, Image-ku, Chiba-shi, Chiba 263-8522, Japan Associate Editor – Vern Manville ABSTRACT Mudrocks are the most common rock type at the Earth’s surface, and they play a major role in informing current understanding of the palaeoenviron- mental history of the planet. Their suitability for this purpose is at least partly underpinned by the assumed stratigraphic completeness of mudrock successions, and the ostensible fidelity with which they record temporal changes in palaeoenvironment. Mud does not necessarily accumulate, how- ever, as a steady, near-continuous ‘rain’ under low energy conditions. Advective modes of mud transport and episodic, ephemeral accumulation have been shown to dominate in many ancient successions. This has impli- cations for the completeness of these records and their suitability for high- resolution sampling and analysis. In this study, a numerical model of mud accumulation, parameterized with data from the Lower Jurassic of Yorkshire (UK) is presented to explore completeness and resolution constraints on ancient epicontinental mudrock successions. Using this model, stratigraphic completeness of the analysed Yorkshire succession is estimated to be ca 13% and ca 98% at centennial and millennial timescales, respectively. The findings indicate that sub-millennial scale processes and events are unlikely to be accurately resolved, despite the largely unbioturbated and well- laminated nature of the succession. -

Mesa Verde National Park Paleontological Resource Inventory (Non-Sensitive Version)
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Mesa Verde National Park Paleontological Resource Inventory (Non-Sensitive Version) Natural Resource Report NPS/MEVE/NRR—2017/1550 ON THE COVER An undescribed chimaera (ratfish) egg capsule of the ichnogenus Chimaerotheca found in the Cliff House Sandstone of Mesa Verde National Park during the work that led to the production of this report. Photograph by: G. William M. Harrison/NPS Photo (Geoscientists-in-the-Parks Intern) Mesa Verde National Park Paleontological Resources Inventory (Non-Sensitive Version) Natural Resource Report NPS/MEVE/NRR—2017/1550 G. William M. Harrison,1 Justin S. Tweet,2 Vincent L. Santucci,3 and George L. San Miguel4 1National Park Service Geoscientists-in-the-Park Program 2788 Ault Park Avenue Cincinnati, Ohio 45208 2National Park Service 9149 79th St. S. Cottage Grove, Minnesota 55016 3National Park Service Geologic Resources Division 1849 “C” Street, NW Washington, D.C. 20240 4National Park Service Mesa Verde National Park PO Box 8 Mesa Verde CO 81330 November 2017 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. -

Structural Styles of the Niobrara Formation: a Study of Kansas and Colorado Outcrops Using an Unmanned Aerial Vehicle (Uav)
STRUCTURAL STYLES OF THE NIOBRARA FORMATION: A STUDY OF KANSAS AND COLORADO OUTCROPS USING AN UNMANNED AERIAL VEHICLE (UAV) by Caleb H. Garbus A thesis submitted to the Faculty and the Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Master of Science (Geology). Golden, Colorado Date Signed: Caleb H. Garbus Signed: Dr. Stephen A. Sonnenberg Thesis Advisor Golden, Colorado Date Signed: Dr. M. Stephen Enders Interim Department Head Department of Geology and Geological Engineering ii ABSTRACT The Late Cretaceous Niobrara Formation is a productive unconventional hydrocarbon system throughout the Denver Basin. Alternating chalk and marl units of the Smoky Hill Member act as a source, reservoir, and seal for the Niobrara petroleum system. The Niobrara has attracted attention from operators after advances in drilling and production technologies have made Niobrara oil and gas economic. The focus of this study aims at achieving a complete understanding of fracture types, fault characteristics, and fault systems in Niobrara outcrop using modern technology. Outcrop analysis across western Kansas indicates the presence of a polygonal fault system (PFS) within the Niobrara. Unmanned aerial vehicle (UAV) data was collected, developed into 3D models, and interpreted to identify fault and joint orientations, geometries, and re- lations across the study area. Polygonal and structurally-reactivated faults were observed in outcrop. Non-tectonic polygonal faults display random orientations, dip-slip normal faults, are characteristically layer-bound, and are unaffected by geomechanical variation. Limited outcrop and exposure bias result in a general SE-NW trend for polygonal strike orienta- tions; however, increased data and hangingwall slip directions confirms the presence of a non-tectonic fault system at Castle Rock, Kansas.