Physics of Energy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AST242 LECTURE NOTES PART 3 Contents 1. Viscous Flows 2 1.1. the Velocity Gradient Tensor 2 1.2. Viscous Stress Tensor 3 1.3. Na
AST242 LECTURE NOTES PART 3 Contents 1. Viscous flows 2 1.1. The velocity gradient tensor 2 1.2. Viscous stress tensor 3 1.3. Navier Stokes equation { diffusion 5 1.4. Viscosity to order of magnitude 6 1.5. Example using the viscous stress tensor: The force on a moving plate 6 1.6. Example using the Navier Stokes equation { Poiseuille flow or Flow in a capillary 7 1.7. Viscous Energy Dissipation 8 2. The Accretion disk 10 2.1. Accretion to order of magnitude 14 2.2. Hydrostatic equilibrium for an accretion disk 15 2.3. Shakura and Sunyaev's α-disk 17 2.4. Reynolds Number 17 2.5. Circumstellar disk heated by stellar radiation 18 2.6. Viscous Energy Dissipation 20 2.7. Accretion Luminosity 21 3. Vorticity and Rotation 24 3.1. Helmholtz Equation 25 3.2. Rate of Change of a vector element that is moving with the fluid 26 3.3. Kelvin Circulation Theorem 28 3.4. Vortex lines and vortex tubes 30 3.5. Vortex stretching and angular momentum 32 3.6. Bernoulli's constant in a wake 33 3.7. Diffusion of vorticity 34 3.8. Potential Flow and d'Alembert's paradox 34 3.9. Burger's vortex 36 4. Rotating Flows 38 4.1. Coriolis Force 38 4.2. Rossby and Ekman numbers 39 4.3. Geostrophic flows and the Taylor Proudman theorem 39 4.4. Two dimensional flows on the surface of a planet 41 1 2 AST242 LECTURE NOTES PART 3 4.5. Thermal winds? 42 5. -

Energy Literacy Essential Principles and Fundamental Concepts for Energy Education
Energy Literacy Essential Principles and Fundamental Concepts for Energy Education A Framework for Energy Education for Learners of All Ages About This Guide Energy Literacy: Essential Principles and Intended use of this document as a guide includes, Fundamental Concepts for Energy Education but is not limited to, formal and informal energy presents energy concepts that, if understood and education, standards development, curriculum applied, will help individuals and communities design, assessment development, make informed energy decisions. and educator trainings. Energy is an inherently interdisciplinary topic. Development of this guide began at a workshop Concepts fundamental to understanding energy sponsored by the Department of Energy (DOE) arise in nearly all, if not all, academic disciplines. and the American Association for the Advancement This guide is intended to be used across of Science (AAAS) in the fall of 2010. Multiple disciplines. Both an integrated and systems-based federal agencies, non-governmental organizations, approach to understanding energy are strongly and numerous individuals contributed to the encouraged. development through an extensive review and comment process. Discussion and information Energy Literacy: Essential Principles and gathered at AAAS, WestEd, and DOE-sponsored Fundamental Concepts for Energy Education Energy Literacy workshops in the spring of 2011 identifies seven Essential Principles and a set of contributed substantially to the refinement of Fundamental Concepts to support each principle. the guide. This guide does not seek to identify all areas of energy understanding, but rather to focus on those To download this guide and related documents, that are essential for all citizens. The Fundamental visit www.globalchange.gov. Concepts have been drawn, in part, from existing education standards and benchmarks. -

Toolkit 1: Energy Units and Fundamentals of Quantitative Analysis
Energy & Society Energy Units and Fundamentals Toolkit 1: Energy Units and Fundamentals of Quantitative Analysis 1 Energy & Society Energy Units and Fundamentals Table of Contents 1. Key Concepts: Force, Work, Energy & Power 3 2. Orders of Magnitude & Scientific Notation 6 2.1. Orders of Magnitude 6 2.2. Scientific Notation 7 2.3. Rules for Calculations 7 2.3.1. Multiplication 8 2.3.2. Division 8 2.3.3. Exponentiation 8 2.3.4. Square Root 8 2.3.5. Addition & Subtraction 9 3. Linear versus Exponential Growth 10 3.1. Linear Growth 10 3.2. Exponential Growth 11 4. Uncertainty & Significant Figures 14 4.1. Uncertainty 14 4.2. Significant Figures 15 4.3. Exact Numbers 15 4.4. Identifying Significant Figures 16 4.5. Rules for Calculations 17 4.5.1. Addition & Subtraction 17 4.5.2. Multiplication, Division & Exponentiation 18 5. Unit Analysis 19 5.1. Commonly Used Energy & Non-energy Units 20 5.2. Form & Function 21 6. Sample Problems 22 6.1. Scientific Notation 22 6.2. Linear & Exponential Growth 22 6.3. Significant Figures 23 6.4. Unit Conversions 23 7. Answers to Sample Problems 24 7.1. Scientific Notation 24 7.2. Linear & Exponential Growth 24 7.3. Significant Figures 24 7.4. Unit Conversions 26 8. References 27 2 Energy & Society Energy Units and Fundamentals 1. KEY CONCEPTS: FORCE, WORK, ENERGY & POWER Among the most important fundamentals to be mastered when studying energy pertain to the differences and inter-relationships among four concepts: force, work, energy, and power. Each of these terms has a technical meaning in addition to popular or colloquial meanings. -

Chapter 30: Quantum Physics ( ) ( )( ( )( ) ( )( ( )( )
Chapter 30: Quantum Physics 9. The tungsten filament in a standard light bulb can be considered a blackbody radiator. Use Wien’s Displacement Law (equation 30-1) to find the peak frequency of the radiation from the tungsten filament. −− 10 1 1 1. (a) Solve Eq. 30-1 fTpeak =×⋅(5.88 10 s K ) for the peak frequency: =×⋅(5.88 1010 s−− 1 K 1 )( 2850 K) =× 1.68 1014 Hz 2. (b) Because the peak frequency is that of infrared electromagnetic radiation, the light bulb radiates more energy in the infrared than the visible part of the spectrum. 10. The image shows two oxygen atoms oscillating back and forth, similar to a mass on a spring. The image also shows the evenly spaced energy levels of the oscillation. Use equation 13-11 to calculate the period of oscillation for the two atoms. Calculate the frequency, using equation 13-1, from the inverse of the period. Multiply the period by Planck’s constant to calculate the spacing of the energy levels. m 1. (a) Set the frequency T = 2π k equal to the inverse of the period: 1 1k 1 1215 N/m f = = = =4.792 × 1013 Hz Tm22ππ1.340× 10−26 kg 2. (b) Multiply the frequency by Planck’s constant: E==×⋅ hf (6.63 10−−34 J s)(4.792 × 1013 Hz) =× 3.18 1020 J 19. The light from a flashlight can be considered as the emission of many photons of the same frequency. The power output is equal to the number of photons emitted per second multiplied by the energy of each photon. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -
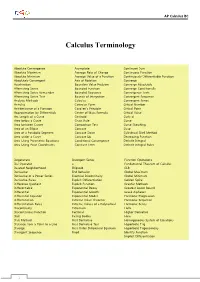
Calculus Terminology
AP Calculus BC Calculus Terminology Absolute Convergence Asymptote Continued Sum Absolute Maximum Average Rate of Change Continuous Function Absolute Minimum Average Value of a Function Continuously Differentiable Function Absolutely Convergent Axis of Rotation Converge Acceleration Boundary Value Problem Converge Absolutely Alternating Series Bounded Function Converge Conditionally Alternating Series Remainder Bounded Sequence Convergence Tests Alternating Series Test Bounds of Integration Convergent Sequence Analytic Methods Calculus Convergent Series Annulus Cartesian Form Critical Number Antiderivative of a Function Cavalieri’s Principle Critical Point Approximation by Differentials Center of Mass Formula Critical Value Arc Length of a Curve Centroid Curly d Area below a Curve Chain Rule Curve Area between Curves Comparison Test Curve Sketching Area of an Ellipse Concave Cusp Area of a Parabolic Segment Concave Down Cylindrical Shell Method Area under a Curve Concave Up Decreasing Function Area Using Parametric Equations Conditional Convergence Definite Integral Area Using Polar Coordinates Constant Term Definite Integral Rules Degenerate Divergent Series Function Operations Del Operator e Fundamental Theorem of Calculus Deleted Neighborhood Ellipsoid GLB Derivative End Behavior Global Maximum Derivative of a Power Series Essential Discontinuity Global Minimum Derivative Rules Explicit Differentiation Golden Spiral Difference Quotient Explicit Function Graphic Methods Differentiable Exponential Decay Greatest Lower Bound Differential -

Calculus Formulas and Theorems
Formulas and Theorems for Reference I. Tbigonometric Formulas l. sin2d+c,cis2d:1 sec2d l*cot20:<:sc:20 +.I sin(-d) : -sitt0 t,rs(-//) = t r1sl/ : -tallH 7. sin(A* B) :sitrAcosB*silBcosA 8. : siri A cos B - siu B <:os,;l 9. cos(A+ B) - cos,4cos B - siuA siriB 10. cos(A- B) : cosA cosB + silrA sirrB 11. 2 sirrd t:osd 12. <'os20- coS2(i - siu20 : 2<'os2o - I - 1 - 2sin20 I 13. tan d : <.rft0 (:ost/ I 14. <:ol0 : sirrd tattH 1 15. (:OS I/ 1 16. cscd - ri" 6i /F tl r(. cos[I ^ -el : sitt d \l 18. -01 : COSA 215 216 Formulas and Theorems II. Differentiation Formulas !(r") - trr:"-1 Q,:I' ]tra-fg'+gf' gJ'-,f g' - * (i) ,l' ,I - (tt(.r))9'(.,') ,i;.[tyt.rt) l'' d, \ (sttt rrJ .* ('oqI' .7, tJ, \ . ./ stll lr dr. l('os J { 1a,,,t,:r) - .,' o.t "11'2 1(<,ot.r') - (,.(,2.r' Q:T rl , (sc'c:.r'J: sPl'.r tall 11 ,7, d, - (<:s<t.r,; - (ls(].]'(rot;.r fr("'),t -.'' ,1 - fr(u") o,'ltrc ,l ,, 1 ' tlll ri - (l.t' .f d,^ --: I -iAl'CSllLl'l t!.r' J1 - rz 1(Arcsi' r) : oT Il12 Formulas and Theorems 2I7 III. Integration Formulas 1. ,f "or:artC 2. [\0,-trrlrl *(' .t "r 3. [,' ,t.,: r^x| (' ,I 4. In' a,,: lL , ,' .l 111Q 5. In., a.r: .rhr.r' .r r (' ,l f 6. sirr.r d.r' - ( os.r'-t C ./ 7. /.,,.r' dr : sitr.i'| (' .t 8. tl:r:hr sec,rl+ C or ln Jccrsrl+ C ,f'r^rr f 9. -

Electrical Power and Energy
Electric Circuits Name: Electrical Power and Energy Read from Lessons 2 and 3 of the Current Electricity chapter at The Physics Classroom: http://www.physicsclassroom.com/Class/circuits/u9l2d.html http://www.physicsclassroom.com/Class/circuits/u9l3d.html MOP Connection: Electric Circuits: sublevel 3 Review: 1. The electric potential at a given location in a circuit is the amount of _____________ per ___________ at that location. The location of highest potential within a circuit is at the _______ ( +, - ) terminal of the battery. As charge moves through the external circuit from the ______ ( +, - ) to the ______ ( +, - ) terminal, the charge loses potential energy. As charge moves through the battery, it gains potential energy. The difference in electric potential between any two locations within the circuit is known as the electric potential difference; it is sometimes called the _____________________ and represented by the symbol ______. The rate at which charge moves past any point along the circuit is known as the ___________________ and is expressed with the unit ________________. The diagram at the right depicts an electric circuit in a car. The rear defroster is connected to the 12-Volt car battery. Several points are labeled along the circuit. Use this diagram for questions #2-#6. 2. Charge flowing through this circuit possesses 0 J of potential energy at point ___. 3. The overall effect of this circuit is to convert ____ energy into ____ energy. a. electrical, chemical b. chemical, mechanical c. thermal, electrical d. chemical, thermal 4. The potential energy of the charge at point A is ___ the potential energy at B. -

Gy Use Units and the Scales of Ener
The Basics: SI Units A tour of the energy landscape Units and Energy, power, force, pressure CO2 and other greenhouse gases conversion The many forms of energy Common sense and computation 8.21 Lecture 2 Units and the Scales of Energy Use September 11, 2009 8.21 Lecture 2: Units and the scales of energy use 1 The Basics: SI Units A tour of the energy landscape Units and Energy, power, force, pressure CO2 and other greenhouse gases conversion The many forms of energy Common sense and computation Outline • The basics: SI units • The principal players:gy ener , power, force, pressure • The many forms of energy • A tour of the energy landscape: From the macroworld to our world • CO2 and other greenhouse gases: measurements, units, energy connection • Perspectives on energy issues --- common sense and conversion factors 8.21 Lecture 2: Units and the scales of energy use 2 The Basics: SI Units tour of the energy landscapeA Units and , force, pressure, powerEnergy CO2 and other greenhouse gases conversion The many forms of energy Common sense and computation SI ≡ International System MKSA = MeterKilogram, , Second,mpereA Unit s Not cgs“English” or units! Electromagnetic units Deriud v n e its ⇒ Char⇒ geCoulombs EnerJ gy oul es ⇒ Current ⇒ Amperes Po werW a tts ⇒ Electrostatic potentialV⇒ olts Pr e ssuP a r s e cals ⇒ Resistance ⇒ Ohms Fo rNe c e wto n s T h erma l un i ts More about these next... TemperatureK⇒ elvinK) ( 8.21 Lecture 2: Units and the scales of energy use 3 The Basics: SI Units A tour of the energy landscape Units and Energy, power, -

3. Energy, Heat, and Work
3. Energy, Heat, and Work 3.1. Energy 3.2. Potential and Kinetic Energy 3.3. Internal Energy 3.4. Relatively Effects 3.5. Heat 3.6. Work 3.7. Notation and Sign Convention In these Lecture Notes we examine the basis of thermodynamics – fundamental definitions and equations for energy, heat, and work. 3-1. Energy. Two of man's earliest observations was that: 1)useful work could be accomplished by exerting a force through a distance and that the product of force and distance was proportional to the expended effort, and 2)heat could be ‘felt’ in when close or in contact with a warm body. There were many explanations for this second observation including that of invisible particles traveling through space1. It was not until the early beginnings of modern science and molecular theory that scientists discovered a true physical understanding of ‘heat flow’. It was later that a few notable individuals, including James Prescott Joule, discovered through experiment that work and heat were the same phenomenon and that this phenomenon was energy: Energy is the capacity, either latent or apparent, to exert a force through a distance. The presence of energy is indicated by the macroscopic characteristics of the physical or chemical structure of matter such as its pressure, density, or temperature - properties of matter. The concept of hot versus cold arose in the distant past as a consequence of man's sense of touch or feel. Observations show that, when a hot and a cold substance are placed together, the hot substance gets colder as the cold substance gets hotter. -

Notes on Thermodynamics the Topic for the Last Part of Our Physics Class
Notes on Thermodynamics The topic for the last part of our physics class this quarter will be thermodynam- ics. Thermodynamics deals with energy transfer processes. The key idea is that materials have "internal energy". The internal energy is the energy that the atoms and molecules of the material possess. For example, in a gas and liquid the molecules are moving and have kinetic energy. The molecules can also rotate and vibrate, and these motions also contribute to the gases total internal energy. In a solid, the atoms can oscillate about their equilibrium position and also possess energy. The total internal energy is defined as: The total internal energy of a substance = the sum of the energies of the constituents of the substance. When two substances come in contact, internal energy from one substance can de- crease while the internal energy of the other increases. The first law of thermody- namics states that the energy lost by one substance is gained by the other. That is, that there exists a quantity called energy that is conserved. We will develop this idea and more over the next 4 weeks. We will be analyzing gases, liquids, and solids, so we need to determine which properties are necessary for an appropriate description of these materials. Although we will be making models about the constituents of these materials, what is usually measured are their macroscopic quantities or "large scale" properties of the sys- tems. We have already some of these when we studied fluids in the beginning of the course: Volume (V ): The volume that the material occupies. -

Best Glide Speed and Distance
General Aviation FAA Joint Steering Committee Aviation Safety Safety Enhancement Topic Best Glide Speed and Distance The General Aviation Joint Steering Committee (GAJSC) has determined that a significant number of general aviation fatalities could be avoided if pilots were better informed and trained in determining and flying their aircraft at the best glide speed while maneuvering to complete a forced landing. What is Best Glide Speed? Keep in mind that this speed will increase with weight so most manufacturers will establish Is it the speed that will get you the greatest the best glide speed at gross weight for the aircraft. distance? Or is it the speed that gets you the That means your best glide speed will be a little longest time in the air? Or are these two the same lower for lower aircraft weights. — the longer you fly, the further you go? Well, as so often is the case, best glide speed depends on what Need More Time? you’re trying to do. If you’re more interested in staying in the air Going the Distance as long as possible to either fix the problem or to communicate your intentions and prepare for a If it’s distance you want, than you’ll need to forced landing, then minimum sink speed is what use the speed and configuration that will get you you’ll need. This speed is rarely found in Pilot the most distance forward for each increment of Operating Handbooks, but it will be a little slower altitude lost. This is often referred to as best glide than maximum glide range speed.