Queso Fresco Cheese Treated with SFE at 120 Bar, 40°C for 30 Minutes For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Price List - Cook School
PRICE LIST - COOK SCHOOL TO PLACE AN ORDER: CALL US ON 01563 55008 OPTION 1 BETWEEN 8AM AND 12PM DAILY, COLLECT FROM 1PM TO 8PM THE SAME DAY. COLLECT FROM BRAEHEAD FOODS WAREHOUSE, TO THE REAR OF BRAEHEAD FOODS/COOK SCHOOL. MINIMUM ORDER VALUE £35, MAXIMUM 1 BOX OF CHICKEN PP. Production Kitchen - Buffet Canape & Starters Product No Product Description UOM Sub Category Column1 Price SHOP041 CS SHOP STEAK & SAUSAGE PIE 1.2 KG EACH £11.00 SHOP048 CCS READY MEAL MINCE POTATOES PEAS AND CARROTS 345 G EACH £3.00 SHOP052 CSS READY MEAL LASAGNE 500G EACH £5.00 SHOP054 CSS READY MEALS CHICKEN BROCCOLI & PASTA BAKE 450 G EACH £4.00 SHOP058 CSS READY MEALS MACARONI CHEESE 400G EACH £3.00 Production Kitchen - Burgers Product No Product Description UOM Sub Category Column1 Price PRO01895 BHF VENISON BURGER 5 x 170g (Frozen) EACH PK Burgers £13.20 Production Kitchen - Hot Wets Product No Product Description UOM Sub Category Column1 Price PRO02003 BHF WHL BEEF & SMOKED PAPRIKA MEATBALLS IN TOMATO SAUCE 2.5Kg (Frozen)EACH Pre 10 PK Hot Wets £28.01 PRO02042 BHF BEEF LASAGNE 2.5Kg (FROZEN) EACH PK Hot Wets £27.64 PRO02015 BHF CAULIFLOWER MAC & CHEESE CRUMBLE 2.5Kg (Frozen) (Vegetarian) Pre 10EACH PK Hot Wets £24.07 PRO02019 BHF CHICK PEA, SQUASH & VEGETABLE CURRY 2.5Kg (Frozen) (Vegetarian) PreEACH 10 PK Hot Wets £21.69 PRO02004 BHF CHICKEN CASSEROLE WITH HERB DUMPLINGS 2.5Kg (Frozen) Pre 10 EACH PK Hot Wets £29.44 PRO02005 BHF CHICKEN TIKKA MASALA CURRY 2.5Kg (Frozen) Pre 10 EACH PK Hot Wets £25.36 PRO02006 BHF CHICKEN, CHICK PEA & CORIANDER TAGINE 2.5Kg -

The Sysco Cheese Product Catalog
> the Sysco Cheese Product Catalog Sysco_Cheese_Cat.indd 1 7/27/12 10:55 AM 5 what’s inside! 4 More Cheese, Please! Sysco Cheese Brands 6 Cheese Trends and Facts Creamy and delicious, 8 Building Blocks... cheese fi ts in with meal of Natural Cheese segments during any Blocks and Shreds time of day – breakfast, Smoked Bacon & Cheddar Twice- Baked Potatoes brunch, lunch, hors d’oeuvres, dinner and 10 Natural Cheese from dessert. From a simple Mild to Sharp Cheddar, Monterey Jack garnish to the basis of and Swiss a rich sauce, cheese is an essential ingredient 9 10 12 A Guide to Great Italian Cheeses Soft, Semi-Soft and for many food service Hard Italian Cheeses operations. 14 Mozzarella... The Quintessential Italian Cheese Slices, shreds, loaves Harvest Vegetable French and wheels… with Bread Pizza such a multitude of 16 Cream Cheese Dreams culinary applications, 15 16 Flavors, Forms and Sizes the wide selection Blueberry Stuff ed French Toast of cheeses at Sysco 20 The Number One Cheese will provide endless on Burgers opportunities for Process Cheese Slices and Loaves menu innovation Stuff ed Burgers and increased 24 Hispanic-Style Cheeses perceived value. Queso Seguro, Special Melt and 20 Nacho Blend Easy Cheese Dip 25 What is Speciality Cheese? Brie, Muenster, Havarti and Fontina Baked Brie with Pecans 28 Firm/Hard Speciality Cheese Gruyère and Gouda 28 Gourmet White Mac & Cheese 30 Fresh and Blue Cheeses Feta, Goat Cheese, Blue Cheese and Gorgonzola Portofi no Salad with 2 Thyme Vinaigrette Sysco_Cheese_Cat.indd 2 7/27/12 10:56 AM welcome. -

Bacteriology of Cheese V. Defects of Blue (Roquefort-Type) Cheese H
Volume 25 Number 283 Bacteriology of cheese V. Defects of blue Article 1 (Roquefort-Type) cheese October 1940 Bacteriology of cheese V. Defects of blue (Roquefort-Type) cheese H. W. Bryant Iowa State College B. W. Hammer Iowa State College Follow this and additional works at: http://lib.dr.iastate.edu/researchbulletin Part of the Agriculture Commons, and the Dairy Science Commons Recommended Citation Bryant, H. W. and Hammer, B. W. (1940) "Bacteriology of cheese V. Defects of blue (Roquefort-Type) cheese," Research Bulletin (Iowa Agriculture and Home Economics Experiment Station): Vol. 25 : No. 283 , Article 1. Available at: http://lib.dr.iastate.edu/researchbulletin/vol25/iss283/1 This Article is brought to you for free and open access by the Iowa Agricultural and Home Economics Experiment Station Publications at Iowa State University Digital Repository. It has been accepted for inclusion in Research Bulletin (Iowa Agriculture and Home Economics Experiment Station) by an authorized editor of Iowa State University Digital Repository. For more information, please contact [email protected]. October, 1940 Research Bulletin 283 Bacteriology of Cheese v. Defects of Blue (Roquefort-Type) Cheese By H. W. BRYANT AND B. W. HAMMER AGRICULTURAL EXPERIMENT STATION IOWA STATE COLLEGE OF AGRICULTURE AND MECHANIC ARTS DAIRY INDUSTRY SECTION • AMES, IOWA • CONTENTS Summary 112 I n trod u c ti 0 n ______ ____________ ______ ____ _______ ______ _____________________________________ _________________ _______ 113 Methods _______________________________ -

Brie/Camembert
“Cheesemaking Made Easy” Brie/Camembert Kate Johnson, The Art of Cheese, LLC www.theartofcheese.com www.facebook.com/artofcheese Equipment Needed: Stainless Steel Pot (1 or 2 Gallon) w/ lid Slotted Spoon Cheese thermometer Large Knife Cheese Form (mold) or Basket Cheese Mat or Tray with holes Dorm refrigerator (for aging your cheese) Ingredients: Fresh or store-bought whole milk, pasteurized (goat’s or cow’s milk) Mesophilic Starter Culture Penicillium candidum mold spores Rennet Cheese salt (or non-iodized sea salt) Ash (optional) To Pasteurize Milk Option 1: Heat milk to 145 degrees stirring constantly. Hold at that temp for 30 minutes. Cool quickly in an ice water bath. Option 2: Heat milk to 161-165 degrees stirring constantly. Hold at that temp for 30 seconds. Cool quickly in an ice water bath. Raw Milk CSA's: www.rawmilkcolorado.org Copyright © 2018 The Art of Cheese www.theartofcheese.com [email protected] Cheesemaking Supply Resources 1. New England Cheesemaking Supply Company, www.cheesemaking.com 2. Artisan Geek, http://artisangeek.com 3. Grape and Granary, www.grapeandgranary.com 4. Hoegger Supply Company, www.thegoatstore.com 5. www.cheesesupply.com Books on Cheesemaking 1. Home Cheese Making by Ricki Carroll 2. 200 Easy Homemade Cheese Recipes by Debra Amrein-Boyes 3. Kitchen Creamery by Louella Hill Lactic Recipes vs Fully Renneted Recipes Lactic Recipes (often rennet-assisted): • Made with little or no rennet and rely primarily on the action of the bacteria converting the milk lactose to lactic acid • Examples: Fresh Mold-Ripened Goat Cheese, Selles sur Cher (Southern French style cheese traditionally made from goat’s milk), Brie de Melun (Northern French lactic acid vs animal rennet cheese considered to be the ancestor of all Bries traditionally made with raw cow’s milk). -

13 Grilled Artichokes, Spanish Goat Cheese, Orange Zest + Mint
OLIVAS TRIO ~ 7 Arbequina, pico-limon + oil cured Moroccan WATERMELON SALAD ~ 14 ALCACHOFAS ~ 13 tomatoes, Tucumcari feta, San Juan honey, purple basil vinaigrette, JAMON IBERICO ~ 26 grilled artichokes, Spanish goat cheese, orange zest + mint * crispy jamon Serrano specialty cured ham made from olive and acorn fed Iberian black CHICARRONES ANDALUZ ~ 9 pigs ~ imported ROMERO FARMS ROMAINE ~ 14 crispy pork belly bites ~ cumin ~ lemon ~ sea salt + harissa cucumber, radish, beets, tomatoes, creamy goat buttermilk and JAMON SERRANO ~ 12 SPICY CARROT GARBANZO HUMMUS ~ 12 dill dressing ham from the sierra’, dry cured Spanish ham ~imported warm parsley butter, house escabeche + sesame crackers MANCHEGO ~ 12 SEARED AHI TUNA SALAD ~ 16 BRUSCHETTA ~ 12 Sheep’s milk cheese from la Mancha region of Spain, membrillo grilled fingerlings, green beans, olives, creamy sweet mustard crimini mushrooms, cream, reggianito, fried egg + truffle oil * dressing, hard boiled eggs LEONORA ~ 12 GAMBAS AL AJILLO ~ 14 goat milk cheese from Leon, Spain, + strawberry honey MUSHROOM MONTADITOS ~ 14 garlic, white wine + chile de arbol red onion jam + quall egg 15 OLD WINDMILL ‘BLUE’ ~ 12 BLACK MUSSELS IN ROMESCO FISH BROTH ~ 14 Local cow’s milk blue cheese with oloroso rosemary figs SPANISH CHICKEN LIVER PATE ~ 12 BACON WRAPPED DATES ~ 12 almonds, ginger oloroso soaked figs + rosemary Stuffed with marcona almond, cabrales dipping sauce + CHORIZOS PALACIOS~ 14 Beer mustard, cornichons pomegranate molasses TUNA CARPACCIO ~ 14 blood orange aioli, black olives, + smoked sea -
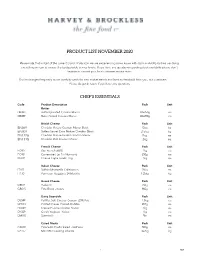
Copy of Product List November 2020
PRODUCT LIST NOVEMBER 2020 Please note that in light of the current Covid-19 situation we are experiencing some issues with stock availability but we are doing everything we can to ensure the best possible service levels. If you have any questions regarding stock availability please don't hesitate to contact your local customer service team. Our list changes frequently as we carefully watch for new market trends and listen to feedback from you, our customers. Please do get in touch if you have any questions. CHEF'S ESSENTIALS Code Product Description Pack Unit Butter DB083 Butter Unsalted Croxton Manor 40x250g ea DB089 Butter Salted Croxton Manor 40x250g ea British Cheese Pack Unit EN069 Cheddar Mature Croxton Manor Block 5kg kg EN003 Butlers Secret Extra Mature Cheddar Block 2.5kg kg EN127G Cheddar Mature Grated Croxton Manor 2kg ea EN131G Cheddar Mild Croxton Manor 2kg ea French Cheese Pack Unit FC417 Brie French (60%) 1kg ea FC431 Camembert Le Fin Normand 250g ea FG021 Chevre Capra Goats' Log 1kg ea Italian Cheese Pack Unit IT042 Buffalo Mozzarella Collebianco 200g ea IT130 Parmesan Reggiano 24 Months 1.25kg kg Greek Cheese Pack Unit GR021 Halloumi 250g ea GR015 Feta Block - Kolios 900g ea Dairy Essentials Pack Unit DS049 Full Fat Soft Cheese Croxton (25% Fat) 1.5kg ea DC033 Clotted Cream Cornish Roddas 907g ea DC049 Crème Fraîche Croxton Manor 2kg ea DY009 Greek Yoghurt - Kolios 1kg ea DM013 Buttermilk 5ltr ea Cured Meats Pack Unit CA049 Prosciutto Crudo Sliced - Dell'ami 500g ea CA177 Mini BBQ Cooking Chorizo 3x2kg kg 1 HBX Chocolate -

Image by Kevin Phillips from Pixabay Who Doesn't Love Cheese? a Nice Dollop of Cheese Can Turn the Most Boring Salad Or Sandwich Into an Exciting Meal
Cheese Comparisons Image by Kevin Phillips from Pixabay Who doesn't love cheese? A nice dollop of cheese can turn the most boring salad or sandwich into an exciting meal. And as with the story of Yehudis, a chunk of cheese can even save a city! Image by HNBS from Pixabay Confused about the many different types out there? Here is some information on the most popular types so you can make better choices. FETA CHEESE Feta cheese is a brine-cured cheese usually made from sheep's milk. The crumbly cheese can also be made with a mixture of sheep and goat's milk and is popularly used in salads, pastries, omelets, sandwiches, or as a garnish. Benefits: 1. Easier to digest: Feta is easier to digest and is less allergenic and inflammatory than most other cheeses, hence a good choice for people who are sensitive to dairy. 2. Lower fat content: Feta cheese is lower in fat and calories as compared to many other types of cheeses. Feta cheese has 21 grams of fat (per 100 grams) as per the USDA. Cheddar on the other hand has 33 grams, while Parmesan has 29 grams of total fat, most of it saturated. 3. Good source of protein: Feta cheese is also a good source of protein, which is immensely beneficial for muscles. 4. Good source of vitamins and minerals: Feta could also be a valuable addition to your diet if you are looking to load up on vitamins from sources other than just fruits. Feta is rich in vitamin B6, vitamin A, as well as the minerals calcium, phosphorous, and potassium, which improve bone health. -

Flavor Description and Classification of Selected Natural Cheeses Delores H
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by K-State Research Exchange Culinary Arts and Sciences V: Global and National Perspectives, 2005, ed. Edwards, J.S.A., Kowrygo, B, & Rejman, K. pp 641-654, Publisher, Worshipful Company of Cooks Research Centre, Bournemouth, Poole, UK Flavor description and classification of selected natural cheeses Delores H. Chambers1, Edgar Chambers IV1 and Dallas Johnson2 1The Sensory Analysis Center, Department of Human Nutrition, Kansas State University, Justin Hall, Manhattan, KS 66506-1407, USA 2Department of Statistics, Kansas State University, Dickens Hall, Manhattan, KS 66506, USA Abstract Intensities of 30 flavor attributes were measured for 42 cheeses. Rated intensities of flavor characteristics generally fell in the low to moderate range for all cheeses. Some of the flavor characteristics (dairy fat, dairy sour, dairy sweet, sharp, astringent, bitter, salty, sour, and sweet) were present in all cheeses, and some (cooked milk, animalic, goaty, fruity, moldy, mushroom, and nutty) were specific to only a few of the cheeses evaluated in this study. The flavor of each of the 42 cheeses is described. Similarities in flavor were observed among many of the individual cheeses. Therefore, a clustering scheme was developed to show the overall flavor relationships among the cheeses. Those relationships are schematically represented by a tree diagram. Proximity on the tree diagram indicates a high degree of flavor similarity among the types of cheese. Introduction In most countries, consumption of cheese has been on the rise over the past decades (Richards, 1989; Magretti, 1996; Havrila, 1997; Hoebermann, 1997; Anonymous, 2002). -
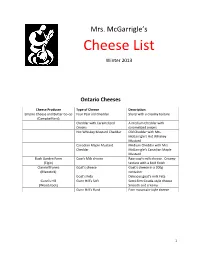
View Cheese List
Mrs. McGarrigle’s Cheese List Winter 2013 Ontario Cheeses Cheese Producer Type of Cheese Description Empire Cheese and Butter Co-op Four Year old Cheddar Sharp with a creamy texture (Campbellford) Cheddar with Caramelized A medium Cheddar with Onions caramelized onions Hot Whiskey Mustard Cheddar Old Cheddar with Mrs. McGarrigle’s Hot Whiskey Mustard Canadian Maple Mustard Medium Cheddar with Mrs. Cheddar McGarrigle’s Canadian Maple Mustard Bush Garden Farm Cow’s Milk cheese Raw cow’s milk cheese. Creamy (Elgin) texture with a bold finish Clarmell Farms Goat’s cheese Goat’s cheese in a 200g (Manotick) container Goat’s Feta Delicious goat’s milk Feta Gunn’s Hill Gunn Hill’s Soft Semi-firm Gouda-style cheese. (Woodstock) Smooth and creamy Gunn Hill’s Hard Firm mountain-style cheese 1 Quebec Cheeses Cheese Producer Type of Cheese Description Alexis de Portneuf La Sauvagine A soft , mixed rind cheese with (Saint Raymond de Portneuf) tastes of fresh butter and mushrooms Calendos A creamy Camembert with a bloomy rind Bleubry A Brie cheese with blue veins. Mild blue flavour St. Honoré Triple cream Brie Laterie Charlevoix Hercule du Charlevoix Semi-firm raw cow’s milk cheese (Baie-Saint-Paul) with nutty, sweet and fruity flavours Blackburn Raw, firm cow’s milk cheese with a Swiss taste Fromagerie Domaine Féodal Le Noble Soft , Brie-style cheese with (Joliette) flavours of mushrooms and almonds Fromagerie Tournevant Chèvre Noir Two year-old goat’s milk (Chesterville) Cheddar L’Abbaye de Saint-Benoit-du-Lac Bleu Bénédictin Semi-soft cow’s milk cheese with (Saint-Benoit-du-Lac) blue veins and a medium blue flavour Le Fromage au Village Cru du Clocher Raw, cow’s milk Cheddar with a (Lorrainville) smooth texture Fromagerie du Presbytère Bleu d’Elizabeth Organic, raw cow’s milk cheese (Sainte-Élizabeth de Warwick) with blue veins. -

2022/23 Complete Wedding Service
2022/23 COMPLETE WEDDING SERVICE 01443 665803 | www.valeresort.com YOUR WEDDING ONE PRICE. EVERYTHING INCLUDED Congratulations, you are about to embark on one of the most memorable events in your life - your wedding. Naturally, nothing less than perfection will do and a whole host of exciting decisions lie before you; the dress, the flowers, the honeymoon and of course, the venue. We’ll help you celebrate the most memorable day of your life in one of the most beautiful wedding venues in Wales. The idyllic setting, exquisite landscaped gardens, sweeping staircases and classic terraces inspire beautiful photography and a day that you’ll cherish and remember for years to come. We cater for your reception with versatile rooms that create the right atmosphere - from intimate, romantic gatherings to show-stopping, lavish celebrations. The stunning Morgannwg Suite opens onto a balcony with sweeping staircases to the terrace below, and beautiful landscaped gardens that provide the ideal backdrop for your photographs. By contrast, the impressive Castle Suite offers a lavish celebration in a dramatic setting with high ceilings, wall to floor windows plus an exclusive bar and balcony terrace for you and your guests. For smaller wedding parties, the Pendoylan Suite, Cowbridge Lounge and Hensol Suite offer the right ambience for intimate gatherings while still guaranteeing a day to remember! 01443 665803 | www.valeresort.com WEDDING CEREMONY Exchanging your vows in a beautiful setting makes those precious words even more special. Our superb choice of function suites enables you to select the perfect setting to say “I do”. Whether your ceremony is an intimate gathering or a celebration for 400 guests, our civil ceremony license allows you to enjoy every aspect of your special day here at the Vale Resort. -

A Guide to Kowalski's Specialty Cheese Read
Compliments of Kowalski’s WWW.KOWALSKIS.COM A GUIDE TO ’ LOCALOUR FAVORITE CHEESES UNDERSTANDING CHEESE TYPES ENTERTAINING WITH CHEESE CHEESE CULTURES OF THE WORLD A PUBLICATION WRITTEN AND PRODUCED BY KOWALSKI’S MARKETS Printed November 2015 SPECIALTY CHEESE EXPERIENCE or many people, Kowalski’s Specialty Cheese Department Sadly, this guide could never be an all-inclusive reference. is their entrée into the world of both cheese and Kowalski’s Clearly there are cheese types and cheesemakers we haven’t Fitself. Many a regular shopper began by exclusively shopping mentioned. Without a doubt, as soon as this guide goes to this department. It’s a tiny little microcosm of the full print, our cheese selection will have changed. We’re certainly Kowalski’s experience, illustrating oh so well our company’s playing favorites. This is because our cheese departments are passion for foods of exceptional character and class. personal – there is an actual person in charge of them, one Cheese Specialist for each and every one of our 10 markets. When it comes to cheese, we pay particular attention Not only do these specialists have their own faves, but so do to cheeses of unique personality and incredible quality, their customers, which is why no two cheese sections look cheeses that are perhaps more rare or have uncommon exactly the same. But though this special publication isn’t features and special tastes. We love cheese, especially local all-encompassing, it should serve as an excellent tool for cheeses, artisanal cheeses and limited-availability treasures. helping you explore the world of cheese, increasing your appreciation and enjoyment of specialty cheese and of that Kowalski’s experience, too. -

Viagra Grapefruit
46 N Jordan Ave 45 Indiana 46 University 45 E Lingelbach Ln E Matlock rd E 10th St N Jefferson St N Fee Ln E Eastgate Ln N Union St Varsity Ln N Forest Ave E 8th St N Woodlawn Ave N Bryan Ave E 17thN Fess St Ave N Indiana Ave N Dunn St N Walnut St W College AveE 15th St Bloomington INNER E 14th St E 3rd St S Mitchell St S Rose Ave S Swan Ave S High St S Jordan Ave S Highland Ave W 16th St S Ballantine Rd W 15th St S Hawthorne Dr W 14th St E 7th St S Faculty Ave Butch’s S Eastside Dr S Woodlawn Ave E Atwater Ave E Hunter Ave S Lincoln St W Kirkwood Ave 12-822-0210 S College Ave S Walnut St E 1st St PHONE: 8 N Adams St FAX: 812-822-0218 S Rogers St WWW.EATBUTCH.COM W 5th St W 3rd St W Smith Ave 120 EAST 7TH STREET BLOOMINGTON, INDIANA 47408 W Howe St W 2nd St W 1st St BIGGER AND BETTER, YOU CAN'T BEAT OUR MEAT At Butch's, we offer a huge selection of the fi nest foods around. We pride ourselves on oversized portions, so be ready for leftovers. See Late night menu for wed - sat 11:00pm - 4:00am Will accomodate orders outside delivery zone for businesses, hotels, and large orders. CATERING Delivery Hours: 12:00 PM - half hour before closing FOR ALL OCCASSIONS PICK UP A CATERING MENU FOR MORE DETAILS MONDAY - TUESDAY 8:00 AM - 1:00 AM DINE IN, CARRY OUT, DELIVERY WEDNESDAY - FRIDAY 8:00 AM - 4:00 AM See Back cover for delivery map and information.