Ibuprofen: Pharmacology, Therapeutics and Side Effects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predicting Toxicity from Gene Expression with Neural Networks
Predicting Toxicity from Gene Expression with Neural Networks Peter Eastman1 and Vijay S. Pande1 1Department of Bioengineering, Stanford University, Stanford, CA 94305 Abstract We train a neural network to predict chemical toxicity based on gene expression data. The input to the network is a full expression profile collected either in vitro from cultured cells or in vivo from live animals. The output is a set of fine grained predictions for the presence of a variety of pathological effects in treated animals. When trained on the Open TG-GATEs database it produces good results, outperforming classical models trained on the same data. This is a promising approach for efficiently screening chemicals for toxic effects, and for more accurately evaluating drug candidates based on preclinical data. Introduction Predicting toxicity is a vital problem in many fields. One quarter of all drug candidates that reach phase II clinical trials ultimately fail because of toxicity[1]. Better methods to predict this in advance would spare patients from taking drugs that ultimately prove toxic, as well as saving enormous time and money. Toxic effects from industrial and household chemicals are also a major public health problem. Often they are tested only on animals, not humans, but animals can be a poor model for toxicity in humans[2]. Better methods to predict human toxicity would have major public health benefits. Many chemicals cause chronic rather than acute toxicity. It may take months or years for their effects to become apparent. Better methods to spot the early signs of chronic toxicity before clinical symptoms appear would allow clinical trials to be stopped sooner, and also would reduce the risk of toxic effects being missed. -

Analysis of Synthetic Chemical Drugs in Adulterated Chinese Medicines by Capillary Electrophoresis/ Electrospray Ionization Mass Spectrometry
RAPID COMMUNICATIONS IN MASS SPECTROMETRY Rapid Commun. Mass Spectrom. 2001; 15: 1473±1480 Analysis of Synthetic chemical drugs in adulterated Chinese medicines by capillary electrophoresis/ electrospray ionization mass spectrometry Hsing Ling Cheng, Mei-Chun Tseng, Pei-Lun Tsai and Guor Rong Her* Department of Chemistry, National Taiwan University, Taipei, Taiwan, R.O.C. Received 20 April 2001; Revised 20 June 2001; Accepted 21 June 2001 Sixteen synthetic chemical drugs, often found in adulterated Chinese medicines, were studied by capillary electrophoresis/UV absorbance CE/UV)and capillary electrophoresis/electrospray ioniza- tion mass spectrometry CE/ESI-MS). Only nine peaks were detected with CZE/UV, but on-line CZE/ MS provided clear identification for most compounds. For a real sample of a Chinese medicinal preparation, a few adulterants were identified by their migration times and protonated molecular ions. For coeluting compounds, more reliable identification was achieved by MS/MS in selected reaction monitoring mode. Micellar electrokinetic chromatography MEKC)using sodium dodecyl sulfate SDS)provided better separation than capillary zone electrophoresis CZE),and, under optimal conditions, fourteen peaks were detected using UV detection. In ESI, the interference of SDS was less severe in positive ion mode than in negative ion mode. Up to 20 mM SDS could be used in direct coupling of MEKC with ESI-MS if the mass spectrometer was operated in positive ion mode. Because of better resolution in MEKC, adulterants can be identified without the use of MS/MS. Copyright # 2001 John Wiley & Sons, Ltd. The use of Chinese medicines to maintain human health and provide the main basis for identification,generally lack the to cure disease has a long and rich history. -

Pharmacological Evaluation of Novel Dimers of an Arylpropionic Acid Class of Non-Selective Cyclooxygenase Inhibitors
Halimi et al Tropical Journal of Pharmaceutical Research February 2017; 16 (2): 327-336 ISSN: 1596-5996 (print); 1596-9827 (electronic) © Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online at http://www.tjpr.org http://dx.doi.org/10.4314/tjpr.v16i2.10 Original Research Article Pharmacological evaluation of novel dimers of an arylpropionic acid class of non-selective cyclooxygenase inhibitors Syed Muhammad Ashhad Halimi1*, Muhammad Saeed1, Safiullah1 and Khalid Muhammed Khan2 1Department of Pharmacy, University of Peshawar, Peshawar 25120, 2H.E.J. International Centre for Chemical and Biological Sciences (ICCBS), University of Karachi, Karachi 75270, Pakistan *For correspondence: Email: [email protected]; Tel: +92 91 9216750; Fax: +92 91 9218318 Received: 4 August 2016 Revised accepted: 5 January 2017 Abstract Purpose: To explore and identify cyclooxygenase (COX) inhibitors with optimal potency and efficacy using an arylpropionic acid class of drugs as lead molecules. Methods: The selected lead molecules were dimerised through chemical processes (reflux condensation) and characterised in terms of structural properties using infrared, proton nuclear magnetic resonance, electron impact mass spectrometry, and elemental analysis techniques. The molecules were evaluated pharmacologically for acute toxicity and anti-inflammatory (carrageenan- induced paw oedema test), analgesic (acetic acid-induced writhing test in mice), and antipyretic (Brewer’s yeast-induced pyrexia test in mice) activities against control (normal saline) and relevant reference standard drugs. Docking analyses were also performed to assess possible protein–ligand interactions. Results: The test compounds were non-toxic at doses of 50, 100 and 150 mg/kg body weight, ip. -

Computational Predictions of Glass-Forming Ability And
Article pubs.acs.org/molecularpharmaceutics Terms of Use Computational Predictions of Glass-Forming Ability and Crystallization Tendency of Drug Molecules † ‡ § † † Amjad Alhalaweh, Ahmad Alzghoul, Waseem Kaialy, Denny Mahlin, and Christel A. S. Bergström*, † Department of Pharmacy, Uppsala University, Uppsala Biomedical Centre, P.O. Box 580, SE-751 23 Uppsala, Sweden ‡ Department of Information Technology, Uppsala University, Lagerhyddsv.̈ 2, hus 1, Box 337, SE-751 05 Uppsala, Sweden § School of Pharmacy, Faculty of Science and Engineering, University of Wolverhampton, Wolverhampton WV1 1LY, United Kingdom ABSTRACT: Amorphization is an attractive formulation technique for drugs suffering from poor aqueous solubility as a result of their high lattice energy. Computational models that can predict the material properties associated with amorphiza- tion, such as glass-forming ability (GFA) and crystallization behavior in the dry state, would be a time-saving, cost-effective, and material-sparing approach compared to traditional experimental procedures. This article presents predictive models of these properties developed using support vector machine (SVM) algorithm. The GFA and crystallization tendency were investigated by melt-quenching 131 drug molecules in situ using differential scanning calorimetry. The SVM algorithm was used to develop computational models based on calculated molecular descriptors. The analyses confirmed the previously suggested cutoff molecular weight (MW) of 300 for glass-formers, and also clarified the extent to which MW can be used to predict the GFA of compounds with MW < 300. The topological equivalent of Grav3_3D, which is related to molecular size and shape, was a better descriptor than MW for GFA; it was able to accurately predict 86% of the data set regardless of MW. -

Ibuprofen Safety at the Golden Anniversary. a Commentary and Recent Developments Giustino Varrassi1,* , Joseph V
Submitted: 08 November, 2020 Accepted: 17 November, 2020 Published: 08 March, 2021 DOI:10.22514/sv.2020.16.0097 EDITORIAL Ibuprofen safety at the golden anniversary. A commentary and recent developments Giustino Varrassi1;* , Joseph V. Pergolizzi2 1Paolo Procacci Foundation, Via Tacito 7, Abstract 00193, Roma, Italy Ibuprofen is a long lasting non-steroidal anti-inflammatory drugs (NSAIDs) and still 2NEMA Research, Inc., Naples, Florida, United States of America represents one of the most diffused analgesics around the world. It has an interesting story started over 50 years ago. In this short comment to an already published paper, *Correspondence the authors try to focus some specific important point. On top, they illustrate the recent, [email protected] confusing and fake assertion on the potentially dangerous influence that ibuprofen could (Giustino Varrassi) have, increasing the risk of Coronavirus infection. This is also better illustrated in a previously published paper, where the readers could find more clear responses to eventual doubts. Keywords NSAIDs; Ibuprofen; COVID-19; Side effects 1. Introduction pathologies or patients’ necessities, but it is indisputable that ibuprofen is safe, and has extremely well-known side effects, The passing of Stewart Adams (Fig. 1) inspired the publica- mainly gastro-intestinal, like any other drug of its class [5]. tion of an interesting paper on the “golden anniversary” of Compared to other OTC analgesics, ibuprofen showed a better ibuprofen [1], whose inventor had been the mentioned great safety profile in a recent study analyzing several medicines Pharmacist [2]. [6]. Also, in a meta-analysis comparing over 1,000 patients He had joined the Boots Pure Drug Co., Ltd. -

COX-1 and COX-2 Enzymes Synthesize Prostaglandins and Are Teacher Emeritus, University of Wisconsin-Madison) Mentor: Dr
COX-1 And COX-2 Enzymes Synthesize Prostaglandins and Are Teacher Emeritus, University of Wisconsin-Madison) Mentor: Dr. David Nelson (Professor of Biochemistry, University of Wisconsin- (Student, University of Wisconsin-Madison) Center for Inhibited by NSAIDS (Nonsteroidal Anti-inflammatory Drugs) BioMolecular Madison West High School: Audra Amasino, Yuting Deng, Samuel Huang, Iris Lee, Adeyinka Lesi, Yaoli Pu, and Peter Vander Velden Modeling Advisor: Gary Graper, Teacher Emeritus, University of Wisconsin-Madison Mentors: Dr. David Nelson, Professor of Biochemistry, and Basudeb Bhattacharyya, Student, University of Wisconsin-Madison Abstract Prostaglandin Hormone Synthases (COX-1 and COX-2) are enzymes that produce prostaglandins. Prostaglandins are (1) Structure (3) Cyclooxygenase Active Sites responsible for fever, pain, and inflammation, but also the (5) Drugs maintenance of the lining of the stomach and prevention of In the pictures below, the heme is orange, the ulceration. COX-1 is found mainly in the gastrointestinal lining, hydrophobic knob is yellow, and the amino acids in the The three drugs below are all nonsteroidal anti- and COX-2 at sites of inflammation. NSAIDS (Nonsteroidal anti- Cyclooxygenase active site are colored in CPK (red for inflammatory drugs (NSAIDS). The first two NSAIDS, inflammatory drugs) such as aspirin, ibuprofen, naproxen, and oxygen, blue for nitrogen, and gray for carbon). aspirin and ibuprofen, are called nonselective Cox flurbiprofen inhibit both COX-1 and COX-2, and are taken inhibitors since they affect both COX-1 and COX-2 regularly by over 33 million Americans for pain and substantially (note their high COX-2/COX-1 effect inflammation. Some 10%-50% of these users suffer ratios). -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] BMJ Open Pediatric drug utilization in the Western Pacific region: Australia, Japan, South Korea, Hong Kong and Taiwan Journal: BMJ Open ManuscriptFor ID peerbmjopen-2019-032426 review only Article Type: Research Date Submitted by the 27-Jun-2019 Author: Complete List of Authors: Brauer, Ruth; University College London, Research Department of Practice and Policy, School of Pharmacy Wong, Ian; University College London, Research Department of Practice and Policy, School of Pharmacy; University of Hong Kong, Centre for Safe Medication Practice and Research, Department -

208271Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 208271Orig1s000 OTHER REVIEW(S) Reference ID: 3963792 Reference ID: 3963792 Reference ID: 3963792 Reference ID: 3963792 Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Office of Medical Policy PATIENT LABELING REVIEW Date: June 22, 2016 To: Donna Griebel, MD Director Division of Gastroenterology and Inborn Errors Products (DGIEP) Through: LaShawn Griffiths, MSHS-PH, BSN, RN Associate Director for Patient Labeling Division of Medical Policy Programs (DMPP) Marcia Williams, PhD Team Leader, Patient Labeling Division of Medical Policy Programs (DMPP) From: Karen Dowdy, RN, BSN Patient Labeling Reviewer Division of Medical Policy Programs (DMPP) Meeta Patel, Pharm.D. Regulatory Review Officer Office of Prescription Drug Promotion (OPDP) Subject: Review of Patient Labeling: Medication Guide (MG) and Instructions for Use (IFU) Drug Name (established RELISTOR (methylnaltrexone bromide) name): Dosage Form and Route: tablets, for oral use injection, for subcutaneous use Application NDA 208271 Type/Number: Applicant: Salix Pharmaceuticals, Inc., a wholly owned subsidiary of Valeant Pharmaceuticals International, Inc., with its affiliate, Valeant Pharmaceutical North America being the communicant Reference ID: 3949385 1 INTRODUCTION On June 19, 2015, Salix Pharmaceuticals, Inc., a wholly owned subsidiary of Valeant Pharmaceuticals International, Inc., with its affiliate, Valeant Pharmaceutical North America being the communicant, submitted for the Agency’s review 505(b)(1) New Drug Application (NDA) 208271 for RELISTOR (methylnaltrexone bromide) tablets. The proposed indication for RELISTOR tablets is for the treatment of opioid- induced constipation (OIC) in adult patients with chronic non-cancer pain. The Applicant cross-references all data contained in RELISTOR Subcutaneous Injection NDA 021964/S-010 approved for the treatment of OIC in adult patients with chronic non-cancer pain on September 29, 2014. -

The Selection and Use of Essential Medicines
WHO Technical Report Series 958 THE SELECTION AND USE OF ESSENTIAL MEDICINES This report presents the recommendations of the WHO Expert THE SELECTION AND USE Committee responsible for updating the WHO Model List of Essential Medicines. The fi rst part contains a review of the OF ESSENTIAL MEDICINES report of the meeting of the Expert Subcommittee on the Selection and Use of Essential Medicines, held in October 2008. It also provides details of new applications for paediatric medicines and summarizes the Committee’s considerations and justifi cations for additions and changes to the Model List, including its recommendations. Part Two of the publication is the report of the second meeting of the Subcommittee of the Expert Committee on the Selection and Use of Essential Medicines. Annexes include the revised version of the WHO Model List of Essential Medicines (the 16th) and the revised version of the WHO Model List of Report of the WHO Expert Committee, 2009 Essential Medicines for Children (the 2nd). In addition there is a list of all the items on the Model List sorted according to their (including the 16th WHO Model List of Essential Medicines Anatomical Therapeutic Chemical (ATC) classifi cation codes. and the 2nd WHO Model List of Essential Medicines for Children) WHO Technical Report Series — 958 WHO Technical ISBN 978-92-4-120958-8 Geneva TTRS958cover.inddRS958cover.indd 1 110.06.100.06.10 008:328:32 The World Health Organization was established in 1948 as a specialized agency of the United Nations serving as the directing and coordinating authority for SELECTED WHO PUBLICATIONS OF RELATED INTEREST international health matters and public health. -

WO 2013/020527 Al 14 February 2013 (14.02.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/020527 Al 14 February 2013 (14.02.2013) P O P C T (51) International Patent Classification: (74) Common Representative: UNIVERSITY OF VETER¬ A61K 9/06 (2006.01) A61K 47/32 (2006.01) INARY AND PHARMACEUTICAL SCIENCES A61K 9/14 (2006.01) A61K 47/38 (2006.01) BRNO FACULTY OF PHARMACY; University of A61K 47/10 (2006.01) A61K 9/00 (2006.01) Veterinary and Pharmaceutical Sciences Brno Faculty Of A61K 47/18 (2006.01) Pharmacy, Palackeho 1/3, CZ-61242 Brno (CZ). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/CZ20 12/000073 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (22) Date: International Filing BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 2 August 2012 (02.08.2012) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (26) Publication Language: English ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (30) Priority Data: NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, 201 1-495 11 August 201 1 ( 11.08.201 1) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, 2012- 72 1 February 2012 (01.02.2012) TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, 2012-5 11 26 July 2012 (26.07.2012) ZW. -

Guidelines for Atcvet Classification 2021
Guidelines for ATCvet classification 2021 ISSN 1020-9891 ISBN 978-82-8406-167-2 Suggested citation: WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATCvet classification 2021. Oslo, 2021. © Copyright WHO Collaborating Centre for Drug Statistics Methodology, Oslo, Norway. Use of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed. Guidelines for ATCvet classification 23rd edition WHO Collaborating Centre for Drug Statistics Methodology Norwegian Institute of Public Health P.O.Box 222 Skøyen N-0213 Oslo Norway Telephone: +47 21078160 E-mail: [email protected] Website: www.whocc.no Previous editions: 1992: Guidelines on ATCvet classification, 1st edition1) 1995: Guidelines on ATCvet classification, 2nd edition1) 1999: Guidelines on ATCvet classification, 3rd edition1) 2002: Guidelines for ATCvet classification, 4th edition2) 2003: Guidelines for ATCvet classification, 5th edition2) 2004: Guidelines for ATCvet classification, 6th edition2) 2005: Guidelines for ATCvet classification, 7th edition2) 2006: Guidelines for ATCvet classification, 8th edition2) 2007: Guidelines for ATCvet classification, 9th edition2) 2008: Guidelines for ATCvet classification, 10th edition2) 2009: Guidelines for ATCvet classification, 11th edition2) 2010: Guidelines for ATCvet classification, 12th edition2) 2011: Guidelines for ATCvet classification, 13th edition2) 2012: -
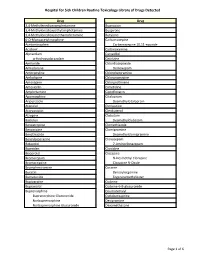
Page 1 S C Routine Toxicology Library Drug 3,4
Hospital for Sick Children Routine Toxicology Library of Drugs Detected Drug Drug 3,4-Methylenedioxyamphetamine Bupropion 3,4-Methylenedioxyethylamphetamine Buspirone 3,4-Methylenedioxymethamphetamine Butylone 6-O-Monoacetylmorphine Carbamazepine Acetaminophen Carbamazepine 10,11-epoxide Aciclovir Carbinoxamine Alprazolam Carvedilol α-Hydroxyalprazolam Cetirizine Amiloride Chlordiazepoxide Amiodarone Demoxepam Amitriptyline Chlorpheniramine Amlodipine Chlorpromazine Amoxapine Chlorprothixene Amoxicillin Cimetidine Amphetamine Ciprofloxacin Apomorphine Citalopram Aripiprazole Desmethylcitalopram Atenolol Clemastine Atorvastatin Clenbuterol Atropine Clobazam Baclofen Desmethylclobazam Benzatropine Clomethiazole Benzocaine Clomipramine Benzthiazide Desmethylclomipramine Benzylpiperazine Clonazepam Betaxolol 7-Aminoclonazepam Biperiden Clonidine Bisoprolol Clozapine Bromazepam N-Desmethyl Clozapine Bromocriptine Clozapine N-Oxide Brompheniramine Cocaine Bucetin Benzoylecgonine Bumetanide Ecgoninemethylester Bupivacaine Codeine Bupranolol Codeine-6-B-glucuronide Buprenorphine Coumatetralyl Buprenorphine Glucuronide Cyclobenzaprine Norbuprenorphine Desipramine Norbuprenorphine Glucuronide Dexamethasone Page 1 of 6 Hospital for Sick Children Routine Toxicology Library of Drugs Detected Drug Drug Dextromethorphan Fluoxetine Dextrorphan Norfluoxetine Diazepam Fluphenazine Nordiazepam Flurazepam 4-Hydroxynordiazepam Desalkylflurazepam Diclofenac 2-Hydroxyethylflurazepam Dihydrocodeine Fluvoxamine Dihydrocodeine-6-β-glucuronide Gabapentin Dihydroergotamine