The Taxonomy of the Genus Hosta and Evolutionary Placement by W
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Liliaceae S.L. (Lily Family)
Liliaceae s.l. (Lily family) Photo: Ben Legler Photo: Hannah Marx Photo: Hannah Marx Lilium columbianum Xerophyllum tenax Trillium ovatum Liliaceae s.l. (Lily family) Photo: Yaowu Yuan Fritillaria lanceolata Ref.1 Textbook DVD KRR&DLN Erythronium americanum Allium vineale Liliaceae s.l. (Lily family) Herbs; Ref.2 Stems often modified as underground rhizomes, corms, or bulbs; Flowers actinomorphic; 3 sepals and 3 petals or 6 tepals, 6 stamens, 3 carpels, ovary superior (or inferior). Tulipa gesneriana Liliaceae s.l. (Lily family) “Liliaceae” s.l. (sensu lato: “in the broad sense”) - Lily family; 288 genera/4950 species, including Lilium, Allium, Trillium, Tulipa; This family is treated in a very broad sense in this class, as in the Flora of the Pacific Northwest. The “Liliaceae” s.l. taught in this class is not monophyletic. It is apparent now that the family should be treated in a narrower sense and some of the members should form their own families. Judd et al. recognize 15+ families: Agavaceae, Alliaceae, Amarylidaceae, Asparagaceae, Asphodelaceae, Colchicaceae, Dracaenaceae (Nolinaceae), Hyacinthaceae, Liliaceae, Melanthiaceae, Ruscaceae, Smilacaceae, Themidaceae, Trilliaceae, Uvulariaceae and more!!! (see web reading “Consider the Lilies”) Iridaceae (Iris family) Photo: Hannah Marx Photo: Hannah Marx Iris pseudacorus Iridaceae (Iris family) Photo: Yaowu Yuan Photo: Yaowu Yuan Sisyrinchium douglasii Sisyrinchium sp. Iridaceae (Iris family) Iridaceae - 78 genera/1750 species, Including Iris, Gladiolus, Sisyrinchium. Herbs, aquatic or terrestrial; Underground stems as rhizomes, bulbs, or corms; Leaves alternate, 2-ranked and equitant Ref.3 (oriented edgewise to the stem; Gladiolus italicus Flowers actinomorphic or zygomorphic; 3 sepals and 3 petals or 6 tepals; Stamens 3; Ovary of 3 fused carpels, inferior. -

The Taxonomic Status of Gladiolus Illyricus (Iridaceae) in Britain
The Taxonomic Status of Gladiolus illyricus (Iridaceae) in Britain Aeron Buchanan Supervisor: Fred Rumsey, Natural History Museum, London A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science of Imperial College, London Abstract First noticed officially in Britain in 1855, Gladiolus illyricus (Koch) presents an interesting taxonomic and biogeographical challenge: whether or not this isolated northern population should be recognized as a separate sub-species. Fundamental conservation issues rest on the outcome. Here, the investigation into the relationship of the G. illyricus plants of the New Forest, Hampshire, to Gladiolus species across Europe, northern Africa and the middle east is initiated. Two chloroplast regions, one in trnL–trnF and the other across psbA–trnH have been sequenced for 42 speci- mens of G. illyricus, G. communis, G. italicus, G. atroviolaceus, G. triphyllos and G. anatolicus. Phylogenetic and biogeographical treatments support the notion of an east–west genetic gradation along the Mediterranean. Iberia particularly appears as a zone of high hybridization potential and the source of the New Forest population. Alignment with sequences obtained from GenBank give strong support to the classic taxonomy of Gladiolus being monophyletic in its sub-family, Ixioideae. Comments on these chloroplast regions for barcoding are also given. In conclusion, the genetic localization of Britain’s G. illyricus population as an extremity haplotype suggests that it could well deserve sub-species status. Contents 1 Introduction 2 2 Background 4 3 Materials and Methods 8 4 Results and Discussion 15 5 Conclusions 26 Appendices 28 References 56 1. Introduction G. illyricus in Britain Figure 1: G. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

G – S C/39/5 ORIGINAL: English/Français/Deutsch/Español DATE/DATUM/FECHA: 2005-10-18
E - F - G – S C/39/5 ORIGINAL: English/français/deutsch/español DATE/DATUM/FECHA: 2005-10-18 INTERNATIONAL UNION UNION INTERNATIONALE INTERNATIONALER UNIÓN INTERNACIONAL FOR THE PROTECTION OF POUR LA PROTECTION VERBAND ZUM SCHUTZ PARA LA PROTECCIÓN NEW VARIETIES DES OBTENTIONS VON PFLANZEN- DE LAS OBTENCIONES OF PLANTS VÉGÉTALES ZÜCHTUNGEN VEGETALES GENEVA GENÈVE GENF GINEBRA COUNCIL CONSEIL DER RAT CONSEJO Thirty-Ninth Ordinary Trente-neuvième session Neununddreißigste ordent- Trigésima novena sesión Session ordinaire liche Tagung ordinaria Geneva, October 27, 2005 Genève, 27 octobre 2005 Genf, 27. Oktober 2005 Ginebra, 27 de octubre de 2005 COOPERATION IN EXAMINATION / COOPÉRATION EN MATIÈRE D’EXAMEN / ZUSAMMENARBEIT BEI DER PRÜFUNG / COOPERACIÓN EN MATERIA DE EXAMEN Document prepared by the Office of the Union / Document établi par le Bureau de l’Union / Vom Verbandsbüro ausgearbeitetes Dokument / Documento preparado por la Oficina de la Unión This document contains a synopsis of offers for cooperation in examination made by authorities, of cooperation already established between authorities and of any envisaged cooperation. * * * * * Le présent document contient une étude synoptique des offres de coopération en matière d’examen faites par les services compétents, de la coopération déjà établie entre des services et de la coopération prévue. * * * * * Dieses Dokument enthält einen Überblick über Angebote für eine Zusammenarbeit bei der Prüfung, die von Behörden abgegeben worden sind, über Fälle einer bereits verwirklichten Zusammenarbeit zwischen Behörden und über Fälle, in denen eine solche Zusammenarbeit beabsichtigt ist. * * * * * Este documento contiene un estudio sinóptico de las ofertas de cooperación en materia de examen realizadas por las autoridades, de la cooperación ya establecida entre autoridades y de cualquier otra cooperación prevista. -

The New Zealand Rain Forest: a Comparison with Tropical Rain Forest! J
The New Zealand Rain Forest: A Comparison with Tropical Rain Forest! J. W. DAWSON2 and B. V. SNEDDON2 ABSTRACT: The structure of and growth forms and habits exhibited by the New Zealand rain forest are described and compared with those of lowland tropical rain forest. Theories relating to the frequent regeneration failure of the forest dominants are outlined. The floristic affinities of the forest type are discussed and it is suggested that two main elements can be recognized-lowland tropical and montane tropical. It is concluded that the New Zealand rain forest is comparable to lowland tropical rain forest in structure and in range of special growth forms and habits. It chiefly differs in its lower stature, fewer species, and smaller leaves. The floristic similarity between the present forest and forest floras of the Tertiary in New Zealand suggest that the former may be a floristically reduced derivative of the latter. PART 1 OF THIS PAPER describes the structure The approximate number of species of seed and growth forms of the New Zealand rain plants in these forests is 240. From north to forest as exemplified by a forest in the far north. south there is an overall decrease in number of In Part 2, theories relating to the regeneration species. At about 38°S a number of species, of the dominant trees in the New Zealand rain mostly trees and shrubs, drop out or become forest generally are reviewed briefly, and their restricted to coastal sites, but it is not until about relevance to the situation in the study forest is 42°S, in the South Island, that many of the con considered. -

Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE
Guide to the Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- LILIACEAE LILIACEAE de Jussieu 1789 (Lily Family) (also see AGAVACEAE, ALLIACEAE, ALSTROEMERIACEAE, AMARYLLIDACEAE, ASPARAGACEAE, COLCHICACEAE, HEMEROCALLIDACEAE, HOSTACEAE, HYACINTHACEAE, HYPOXIDACEAE, MELANTHIACEAE, NARTHECIACEAE, RUSCACEAE, SMILACACEAE, THEMIDACEAE, TOFIELDIACEAE) As here interpreted narrowly, the Liliaceae constitutes about 11 genera and 550 species, of the Northern Hemisphere. There has been much recent investigation and re-interpretation of evidence regarding the upper-level taxonomy of the Liliales, with strong suggestions that the broad Liliaceae recognized by Cronquist (1981) is artificial and polyphyletic. Cronquist (1993) himself concurs, at least to a degree: "we still await a comprehensive reorganization of the lilies into several families more comparable to other recognized families of angiosperms." Dahlgren & Clifford (1982) and Dahlgren, Clifford, & Yeo (1985) synthesized an early phase in the modern revolution of monocot taxonomy. Since then, additional research, especially molecular (Duvall et al. 1993, Chase et al. 1993, Bogler & Simpson 1995, and many others), has strongly validated the general lines (and many details) of Dahlgren's arrangement. The most recent synthesis (Kubitzki 1998a) is followed as the basis for familial and generic taxonomy of the lilies and their relatives (see summary below). References: Angiosperm Phylogeny Group (1998, 2003); Tamura in Kubitzki (1998a). Our “liliaceous” genera (members of orders placed in the Lilianae) are therefore divided as shown below, largely following Kubitzki (1998a) and some more recent molecular analyses. ALISMATALES TOFIELDIACEAE: Pleea, Tofieldia. LILIALES ALSTROEMERIACEAE: Alstroemeria COLCHICACEAE: Colchicum, Uvularia. LILIACEAE: Clintonia, Erythronium, Lilium, Medeola, Prosartes, Streptopus, Tricyrtis, Tulipa. MELANTHIACEAE: Amianthium, Anticlea, Chamaelirium, Helonias, Melanthium, Schoenocaulon, Stenanthium, Veratrum, Toxicoscordion, Trillium, Xerophyllum, Zigadenus. -

Revised Glossary for AQA GCSE Biology Student Book
Biology Glossary amino acids small molecules from which proteins are A built abiotic factor physical or non-living conditions amylase a digestive enzyme (carbohydrase) that that affect the distribution of a population in an breaks down starch ecosystem, such as light, temperature, soil pH anaerobic respiration respiration without using absorption the process by which soluble products oxygen of digestion move into the blood from the small intestine antibacterial chemicals chemicals produced by plants as a defence mechanism; the amount abstinence method of contraception whereby the produced will increase if the plant is under attack couple refrains from intercourse, particularly when an egg might be in the oviduct antibiotic e.g. penicillin; medicines that work inside the body to kill bacterial pathogens accommodation ability of the eyes to change focus antibody protein normally present in the body acid rain rain water which is made more acidic by or produced in response to an antigen, which it pollutant gases neutralises, thus producing an immune response active site the place on an enzyme where the antimicrobial resistance (AMR) an increasing substrate molecule binds problem in the twenty-first century whereby active transport in active transport, cells use energy bacteria have evolved to develop resistance against to transport substances through cell membranes antibiotics due to their overuse against a concentration gradient antiretroviral drugs drugs used to treat HIV adaptation features that organisms have to help infections; they -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -
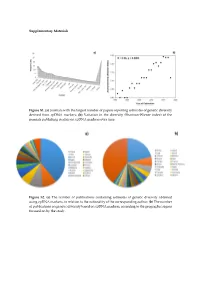
(A) Journals with the Largest Number of Papers Reporting Estimates Of
Supplementary Materials Figure S1. (a) Journals with the largest number of papers reporting estimates of genetic diversity derived from cpDNA markers; (b) Variation in the diversity (Shannon-Wiener index) of the journals publishing studies on cpDNA markers over time. Figure S2. (a) The number of publications containing estimates of genetic diversity obtained using cpDNA markers, in relation to the nationality of the corresponding author; (b) The number of publications on genetic diversity based on cpDNA markers, according to the geographic region focused on by the study. Figure S3. Classification of the angiosperm species investigated in the papers that analyzed genetic diversity using cpDNA markers: (a) Life mode; (b) Habitat specialization; (c) Geographic distribution; (d) Reproductive cycle; (e) Type of flower, and (f) Type of pollinator. Table S1. Plant species identified in the publications containing estimates of genetic diversity obtained from the use of cpDNA sequences as molecular markers. Group Family Species Algae Gigartinaceae Mazzaella laminarioides Angiospermae Typhaceae Typha laxmannii Angiospermae Typhaceae Typha orientalis Angiospermae Typhaceae Typha angustifolia Angiospermae Typhaceae Typha latifolia Angiospermae Araliaceae Eleutherococcus sessiliflowerus Angiospermae Polygonaceae Atraphaxis bracteata Angiospermae Plumbaginaceae Armeria pungens Angiospermae Aristolochiaceae Aristolochia kaempferi Angiospermae Polygonaceae Atraphaxis compacta Angiospermae Apocynaceae Lagochilus macrodontus Angiospermae Polygonaceae Atraphaxis -

Dr JL Birch School of Biosciences the University of Melbourne Parkville, VIC 3010 E-Mail
Dr J.L. Birch School of BioSciences The University of Melbourne Parkville, VIC 3010 e-mail: [email protected] Phone: +613-8344-5040 EMPLOYMENT Herbarium Curator and Lecturer The University of Melbourne Herbarium (MELU) June 2016-current School of Biosciences Post-Doctoral Fellow Royal Botanic Gardens Victoria. November 2011-May 2016 Supervisor: Dr Daniel J. Murphy Project Title: Systematics of Australian tussock grasses (Poaceae tribe Poeae) EDUCATION Ph.D. Botany Department and Ecology, Evolution, and Conservation Biology, University of Hawaii at Manoa (UHM). August 2011 Advisor: Dr Clifford W. Morden Dissertation title: The systematics and historical biogeography of the Asteliaceae (Asparagales) Master's Plant Biology, University of Texas at Austin (UTA). August 2004 Advisor: Dr Beryl B. Simpson Thesis title: The Gideon Lincecum Herbarium: A Floristic and Ethnobotanic Analysis B.S. Plant Biology, University of Texas at Austin. August 2001 B.S. Botany, Victoria University of Wellington. December 1990 RESEARCH Research Grants (selected): (TOTAL: $514,000 AUD) 2016 Hermon Slade Foundation ($81,000 AUD) 2016 Australian Biological Resources Study Post-doctoral Fellowship Grant ($270,000 AUD) 2015 Australian Biological Resources Study Applied Taxonomy Grant ($88,000 AUD) 2013 Cuatrecasas Travel Fellowship, Smithsonian Institution ($700 AUD/$500 USD) 2011 Association of Plant Taxonomists, IBC Graduate Student Travel Award ($2800 AUD/$2,000 USD) 2011 Graduate Student Organization (UHM), Graduate Student Travel Grant (($2800 AUD/$2,000 USD) 2010 Graduate Division (UHM), RCUH Dissertation Completion Award ($12600 AUD/$9,000 USD) 2009 National Science Foundation, Doctoral Dissertation Improvement Grant ($7500 AUD/$5,350 USD) 2009 Evolution, Ecology, and Conservation Biology Program (UHM), Watson T. -

Congolius, a New Genus of African Reed Frog Endemic to The
www.nature.com/scientificreports OPEN Congolius, a new genus of African reed frog endemic to the central Congo: A potential case of convergent evolution Tadeáš Nečas1,2*, Gabriel Badjedjea3, Michal Vopálenský4 & Václav Gvoždík1,5* The reed frog genus Hyperolius (Afrobatrachia, Hyperoliidae) is a speciose genus containing over 140 species of mostly small to medium-sized frogs distributed in sub-Saharan Africa. Its high level of colour polymorphism, together with in anurans relatively rare sexual dichromatism, make systematic studies more difcult. As a result, the knowledge of the diversity and taxonomy of this genus is still limited. Hyperolius robustus known only from a handful of localities in rain forests of the central Congo Basin is one of the least known species. Here, we have used molecular methods for the frst time to study the phylogenetic position of this taxon, accompanied by an analysis of phenotype based on external (morphometric) and internal (osteological) morphological characters. Our phylogenetic results undoubtedly placed H. robustus out of Hyperolius into a common clade with sympatric Cryptothylax and West African Morerella. To prevent the uncovered paraphyly, we place H. robustus into a new genus, Congolius. The review of all available data suggests that the new genus is endemic to the central Congolian lowland rain forests. The analysis of phenotype underlined morphological similarity of the new genus to some Hyperolius species. This uniformity of body shape (including cranial shape) indicates that the two genera have either retained ancestral morphology or evolved through convergent evolution under similar ecological pressures in the African rain forests. African reed frogs, Hyperoliidae Laurent, 1943, are presently encompassing almost 230 species in 17 genera.