Appendix a – Data for Sample Sites, Avra Valley Sub-Basin, 1998-2001
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chiricahua Leopard Frog (Rana Chiricahuensis)
U.S. Fish & Wildlife Service Chiricahua Leopard Frog (Rana chiricahuensis) Final Recovery Plan April 2007 CHIRICAHUA LEOPARD FROG (Rana chiricahuensis) RECOVERY PLAN Southwest Region U.S. Fish and Wildlife Service Albuquerque, New Mexico DISCLAIMER Recovery plans delineate reasonable actions that are believed to be required to recover and/or protect listed species. Plans are published by the U.S. Fish and Wildlife Service, and are sometimes prepared with the assistance of recovery teams, contractors, state agencies, and others. Objectives will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Recovery plans do not necessarily represent the views nor the official positions or approval of any individuals or agencies involved in the plan formulation, other than the U.S. Fish and Wildlife Service. They represent the official position of the U.S. Fish and Wildlife Service only after they have been signed by the Regional Director, or Director, as approved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery tasks. Literature citation of this document should read as follows: U.S. Fish and Wildlife Service. 2007. Chiricahua Leopard Frog (Rana chiricahuensis) Recovery Plan. U.S. Fish and Wildlife Service, Southwest Region, Albuquerque, NM. 149 pp. + Appendices A-M. Additional copies may be obtained from: U.S. Fish and Wildlife Service U.S. Fish and Wildlife Service Arizona Ecological Services Field Office Southwest Region 2321 West Royal Palm Road, Suite 103 500 Gold Avenue, S.W. -
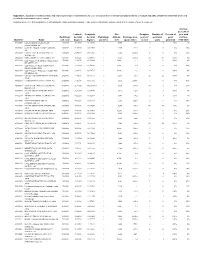
USGS Open-File Report 2009-1269, Appendix 1
Appendix 1. Summary of location, basin, and hydrological-regime characteristics for U.S. Geological Survey streamflow-gaging stations in Arizona and parts of adjacent states that were used to calibrate hydrological-regime models [Hydrologic provinces: 1, Plateau Uplands; 2, Central Highlands; 3, Basin and Range Lowlands; e, value not present in database and was estimated for the purpose of model development] Average percent of Latitude, Longitude, Site Complete Number of Percent of year with Hydrologic decimal decimal Hydrologic altitude, Drainage area, years of perennial years no flow, Identifier Name unit code degrees degrees province feet square miles record years perennial 1950-2005 09379050 LUKACHUKAI CREEK NEAR 14080204 36.47750 109.35010 1 5,750 160e 5 1 20% 2% LUKACHUKAI, AZ 09379180 LAGUNA CREEK AT DENNEHOTSO, 14080204 36.85389 109.84595 1 4,985 414.0 9 0 0% 39% AZ 09379200 CHINLE CREEK NEAR MEXICAN 14080204 36.94389 109.71067 1 4,720 3,650.0 41 0 0% 15% WATER, AZ 09382000 PARIA RIVER AT LEES FERRY, AZ 14070007 36.87221 111.59461 1 3,124 1,410.0 56 56 100% 0% 09383200 LEE VALLEY CR AB LEE VALLEY RES 15020001 33.94172 109.50204 1 9,440e 1.3 6 6 100% 0% NR GREER, AZ. 09383220 LEE VALLEY CREEK TRIBUTARY 15020001 33.93894 109.50204 1 9,440e 0.5 6 0 0% 49% NEAR GREER, ARIZ. 09383250 LEE VALLEY CR BL LEE VALLEY RES 15020001 33.94172 109.49787 1 9,400e 1.9 6 6 100% 0% NR GREER, AZ. 09383400 LITTLE COLORADO RIVER AT GREER, 15020001 34.01671 109.45731 1 8,283 29.1 22 22 100% 0% ARIZ. -

Historical Geomorphology .. and Hydrology of the Santa Cruz River
Historical Geomorphology .. and Hydrology of the Santa Cruz River Michelle Lee Wood, P. Kyle House, and Philip A. Pearthree Arizona Geological Survey Open-File Report 99-13 July 1999 Text 98 p., 1 sheet, scale 1: 100,000 Investigations supported by the Arizona State Land Department as part of their efforts to gather technical information for a stream navigability assessment This report is preliminary and has not been edited or reviewed for conformity with Arizona Geological Survey standards. EXTENDED ABSTRACT This report provides baseline information on the physical characteristics of the Santa Cruz River to be used by the Arizona Stream Navigability Commission in its determination of the potential navigability of the Santa Cruz River at the time of Statehood. The primary goals of this report are: (1) to give a descriptive overview of the geography, geology, climatology, vegetation and hydrology that define the character of the Santa Cruz River; and, (2) to describe how the character of the Santa Cruz River has changed since the time of Statehood with special focus on the streamflow conditions and geomorphic changes such as channel change and movement. This report is based on a review of the available literature and analyses of historical survey maps, aerial photographs, and U.S. Geological Survey streamgage records. The Santa Cruz River has its source at the southern base of the Canelo Hills in the Mexican Highlands portion of the Basin and Range province. The river flows south through the San Rafael Valley before crossing the international border into Mexico. It describes a loop of about 30 miles before it re-enters the United States six miles east of Nogales, and continues northward past Tucson to its confluence with the Gila River a few miles above the mouth of the Salt River. -

Geochemistry of Ground Water in Avra Valley, Pima County, Arizona
Geochemistry of ground water in Avra Valley, Pima County, Arizona Item Type Thesis-Reproduction (electronic); text Authors Conner, Leslee Lynn,1957- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 01/10/2021 12:48:22 Link to Item http://hdl.handle.net/10150/191892 GEOCHEMISTRY OF GROUND WATER IN AVRA VALLEY, PIMA COUNTY, ARIZONA by Leslee Lynn Conner A Thesis Submitted to the Faculty of the DEPARTMENT OF HYDROLOGY AND WATER RESOURCES In Partial Fulfillment of the Requirements For the Degree of MASTER OF SCIENCE WITH A MAJOR IN HYDROLOGY In the Graduate College THE UNIVERSITY OF ARIZONA 1986 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfillment of re- quirements for an advanced degree at The University of Arizona and is deposited in the University Library to be made available to borrowers under rules of the Library. Brief quotations from this thesis are allowable without special permission, provided that accurate acknowledgment of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in his or her judgment the proposed use of the material is in the interests of scholarship. In all other instances, however, permission must be obtained by the author. -

A STRUCTURAL and GEOCHEMICAL STUDY of the SIERRITA PORPHYRY COPPER SYSTEM, PIMA COUNTY, ARIZONA Paul William Jensen a Thesis
A STRUCTURAL AND GEOCHEMICAL STUDY OF THE SIERRITA PORPHYRY COPPER SYSTEM, PIMA COUNTY, ARIZONA by Paul William Jensen A Thesis Submitted to the Faculty of the DEPARTMENT OF GEOSCIENCES In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 1998 2 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfillment of requirements for an advanced degree at the University of Arizona and is deposited in the University Library to be made available to borrowers under rules of the Library. Brief quotations from this thesis are allowable without special permission, provided that accurate acknowledgment of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in his or her judgment the proposed use of the material is in the interests of scholarship. In all other instances however, permission must be obtained from the author. SIGNED: APPROVAL BY THESIS DIRECTOR This thesis has been approved on the date shown below: -1 ., ./lG+ .. /lf j^.S/ /`¡ÿ FÇ Spencer R. Titley Date// Professor of Geosciences 2 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfillment of requirementsfor an advanced degree at the University of Arizona and is deposited inthe University Library to be made available to borrowers under rules of the Library. Brief quotations from this thesis are allowable without specialpermission, provided that accurate acknowledgment of source is made. Requestsfor permission for extended quotation from or reproduction of this manuscript in whole orin part may be granted by the head of the major department or the Dean of the GraduateCollege when in his or her judgment the proposed use of the material is in the interests ofscholarship. -

Canoa Ranch Groundwater Evaluation
Canoa Ranch Groundwater Evaluation Hydrologic Evaluation of the Occurrence and Nature of Groundwater, and Water Level Trends and Water Balance Related to Future Water Resource Use in the Canoa Ranch Area, Upper Santa Cruz Basin, Pima County, Arizona By Frank G. Postillion, C.G.W.P. Chief Hydrologist David Scalero, Principal Hydrologist Mark Krieski, P.E. Engineering Manager Pima County Regional Flood Control District Executive Summary The Water Resources Division of the Pima County Regional Flood Control District has conducted a hydrologic evaluation of the Canoa Ranch and vicinity, with a focus on the historical and current water balance and how it has affected local groundwater- level conditions, and the potential impacts on the hydro- and meso-riparian vegetation of the Ranch. Numerous data sources were evaluated during the study, including the U.S. Geological Survey (USGS), Arizona Department of Water Resources (ADWR), Pima County, Pima Association of Governments, and consulting reports. The Canoa Ranch hydrologic study area is centered along the Santa Cruz River (SCR) from the Santa Cruz/Pima County Line in Pima County, Arizona to about 7.5 miles downstream along the River (Figure 1-1). Large portions of the study area along the SCR (4,800 acres) are owned by Pima County as part of the old Canoa Ranch; Elephant Head Road from the South to Santa Rita Springs Development and the Farmers Investment Company (FICO) on the north; from Interstate 19 on the west to the floodplain pediments on the east. The historic Canoa Ranch is undergoing building restoration and is slated for riparian restoration in some targeted areas. -

1 U.S. Department of the Interior Bureau of Land Management
U.S. Department of the Interior Bureau of Land Management Keystone Peak Prescribed Burn Environmental Assessment DOI-BLM-AZ-G020-2015-0009-EA 1 Table of Contents 1 INTRODUCTION .............................................................................................................. 8 1.1 Background ............................................................................................................................................. 8 1.2 Purpose and Need .................................................................................................................................... 9 1.3 Decision to be Made .............................................................................................................................. 11 1.4 Conformance with Land Use Plan .......................................................................................................... 11 1.5 Scoping and Issues ................................................................................................................................ 12 1.5.1 Internal Scoping ................................................................................................................................ 12 1.5.2 External Scoping ............................................................................................................................... 12 1.5.3 Issues Identified ................................................................................................................................ 12 2 DESCRIPTION OF ALTERNATIVES ............................................................................ -

THE ARCHAIC OCCUPATION of the ROSEMONT AREA, NORTHERN SANTA RITA MOUNTAINS, SOUTHEASTERN ARIZONA by Bruce B. Huckell K with Cont
THE ARCHAIC OCCUPATION OF THE ROSEMONT AREA, NORTHERN SANTA RITA MOUNTAINS, SOUTHEASTERN ARIZONA by Bruce B. Huckell K with contributions by Lisa W. Huckell Robert S. Thompson Cultural Resource Management Division Arizona State Museum University of Arizona Archaeological Series No. 147, Vol. I THE ARCHAIC OCCUPATION OF THE ROSEMONT AREA, NORTHERN SANTA RITA MOUNTAINS, SOUTHEASTERN ARIZONA by Bruce B. Huckell Contributions by Lisa W. Huckell Robert S. Thompson Submitted by Cultural Resource Management Division Arizona State Museum University of Arizona Prepared for ANAMAX Mining Company 1984 Archaeological Series No. 147, Vol. I CONTENTS FIGURES vii TABLES PREFACE xiii ACKNOWLEDGMENTS xvi ABSTRACT xviii Chapter 1. INTRODUCTION 1 The Archaic Period 2 Previous Research 5 2. THE ENVIRONMENT OF THE ROSEMONT AREA AND SURROUNDING REGIONS 11 General Geography 11 Geology 13 Climate 17 Vegetation 19 Fauna 28 The Paleoenvironment 29 Recent Changes in the Local Biotic Communities 30 Long-Term Changes in the Environment 32 3. A FRAMEWORK FOR RESEARCH AT THE ROSEMONT ARCHAIC SITES 35 Hunter-Gatherer Organization 35 Research Domains 38 Problem Domain I: Subsistence-Settlement Systems 38 Problem Domain II: Cultural and Temporal Affinities 40 Site Selection 41 4. INVESTIGATIONS AT AND DESCRIPTIONS OF THE SITES 43 Field Methods 43 Results of Excavations: Site Descriptions 46 AZ EE:2:62--The Wasp Canyon Site 48 Investigation 50 Features 52 Structures 52 Rock Clusters 56 Artifacts 58 iii iv Contents AZ EE:2:65 59 Investigation 59 -

Hohokam Reservoirs, Tucson Presidio
ARCHAEOLOGY IN TUCSON Vol. 6, No. 4 Newsletter of the Center for Desert Archaeology October 1992 HOHOKAM RESERVOIRS AND THEIR ROLE IN AN ANCIENT DESERT ECONOMY By James M. Bayman Desert Archaeology, Inc. Papaguería—the vicinity of the Tohono O'odham Indian Reservation), Arizona's most famous archaeologist, Emil W. Haury, even referred to the people of these drier areas as the ''Desert Branch Hohokam." Until fairly recently, Hohokam populations in these nonriverine areas were perceived to be of secondary importance in greater Hohokam society, and the Phoenix Basin was long viewed as an economic center for prehistoric central and southern Arizona (much as it is today). A common explanation for the apparent contrast between the Phoenix Hohokam and "everybody else" was that canal irrigation systems required an advanced organizational system, in other words, "people in control," for their design, construction, and maintenance. Moreover, because large- scale canal irrigation was simply not feasible in Hohokam Figure 1. A modern "charco," or reservoir, in the settlements outside the greater Phoenix area, it was thought Baboquivari Valley, at the modern village of Ali Chukson on that the ''Desert Branch" Hohokam never developed and the Tohono O’odham Reservation. This charco may be quite flourished to the extent of their neighbors. similar to prehistoric reservoirs utilized by the Hohokam in the nonriverine areas of southern Arizona. Photo by Jonathan A tremendous amount of recent archaeological survey and B. Mabry. excavation, however, has produced evidence that the Hohokam "regional system" was more diverse than previously thought. In the vast deserts between Tucson and Phoenix studies by institutions like Arizona State University, The remarkable development and desert adaptation of the the Arizona State Museum (University of Arizona), and the prehistoric Hohokam has long impressed explorers, settlers, Museum of Northern Arizona have resulted in the discovery and later, archaeologists who came to live and work in arid of a number of extremely large Hohokam sites. -

Department of the Interior
Vol. 77 Tuesday, No. 54 March 20, 2012 Part II Department of the Interior Fish and Wildlife Service 50 CFR Part 17 Endangered and Threatened Wildlife and Plants; Listing and Designation of Critical Habitat for the Chiricahua Leopard Frog; Final Rule VerDate Mar<15>2010 16:12 Mar 19, 2012 Jkt 226001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\20MRR2.SGM 20MRR2 tkelley on DSK3SPTVN1PROD with RULES2 16324 Federal Register / Vol. 77, No. 54 / Tuesday, March 20, 2012 / Rules and Regulations DEPARTMENT OF THE INTERIOR Background submitting the critical habitat rules to the Federal Register. Fish and Wildlife Service It is our intent to discuss in this final We published a proposed rule to rule only those topics directly relevant reassess the listing status and propose 50 CFR Part 17 to the listing and development and critical habitat for the Chiricahua designation of critical habitat for the leopard frog in the Federal Register on [Docket No. FWS–R2–ES–2010– Chiricahua leopard frog under the Act 0085;4500030114] March 15, 2011 (76 FR 14126) with a (16 U.S.C. 1531 et seq.). For more request for public comments. On RIN 1018–AX12 information on the biology and ecology September 21, 2011, we made available of the Chiricahua leopard frog refer to the draft environmental assessment and Endangered and Threatened Wildlife the final listing rule (67 FR 40790; June draft economic analysis for the and Plants; Listing and Designation of 13, 2002) or our April 2007 final proposed designation of critical habitat Critical Habitat for the Chiricahua recovery plan, which are available from and reopened the public comment on Leopard Frog the Arizona Ecological Services Field the proposed rule (76 FR 58441). -

Feasibility Study for the SANTA CRUZ VALLEY NATIONAL HERITAGE AREA
Feasibility Study for the SANTA CRUZ VALLEY NATIONAL HERITAGE AREA FINAL Prepared by the Center for Desert Archaeology April 2005 CREDITS Assembled and edited by: Jonathan Mabry, Center for Desert Archaeology Contributions by (in alphabetical order): Linnea Caproni, Preservation Studies Program, University of Arizona William Doelle, Center for Desert Archaeology Anne Goldberg, Department of Anthropology, University of Arizona Andrew Gorski, Preservation Studies Program, University of Arizona Kendall Kroesen, Tucson Audubon Society Larry Marshall, Environmental Education Exchange Linda Mayro, Pima County Cultural Resources Office Bill Robinson, Center for Desert Archaeology Carl Russell, CBV Group J. Homer Thiel, Desert Archaeology, Inc. Photographs contributed by: Adriel Heisey Bob Sharp Gordon Simmons Tucson Citizen Newspaper Tumacácori National Historical Park Maps created by: Catherine Gilman, Desert Archaeology, Inc. Brett Hill, Center for Desert Archaeology James Holmlund, Western Mapping Company Resource information provided by: Arizona Game and Fish Department Center for Desert Archaeology Metropolitan Tucson Convention and Visitors Bureau Pima County Staff Pimería Alta Historical Society Preservation Studies Program, University of Arizona Sky Island Alliance Sonoran Desert Network The Arizona Nature Conservancy Tucson Audubon Society Water Resources Research Center, University of Arizona PREFACE The proposed Santa Cruz Valley National Heritage Area is a big land filled with small details. One’s first impression may be of size and distance—broad valleys rimmed by mountain ranges, with a huge sky arching over all. However, a closer look reveals that, beneath the broad brush strokes, this is a land of astonishing variety. For example, it is comprised of several kinds of desert, year-round flowing streams, and sky island mountain ranges. -

Simulation of Ground-Water Flow and Potential Land Subsidence, Avra Valley, Arizona
SIMULATION OF GROUND-WATER FLOW AND POTENTIAL LAND SUBSIDENCE, AVRA VALLEY, ARIZONA By R.T. HANSON, S.R. ANDERSON, and D.R. POOL U.S. GEOLOGICAL SURVEY Water-Resources Investigations Report 90-4178 Prepared in cooperation with the CITY OF TUCSON Tucson, Arizona December 1990 U.S DEPARTMENT OF THE INTERIOR MANUEL LUJAN, JR., Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director For additional information Copies of this report can be write to: purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Books and Open-File Reports Section Federal Building, FB-44 Federal Center, Building 810 300 West Congress Street Box 25425 Tucson, Arizona 85701-1393 Denver, Colorado 80225 CONTENTS Page Abstract.......................................................... 1 Introduction...................................................... 1 Hydrogeologic setting............................................. 3 Geology...................................................... 4 Aquifer system............................................... 6 Ground-water development..................................... 9 Simulation of ground-water flow................................... 11 Steady-state simulation...................................... 13 Transient-state simulation................................... 18 Sensitivity analysis......................................... 28 Simulation of potential land subsidence........................... 28 Summary........................................................... 35 Selected references..............................................